
Evaluation of 4 different initial doses of envarsus in renal transplant recipients de novo. Is there overdose?
Antonio Franco Esteve1,4, Patricio Más-Serrano2,3,4, Ivan Beltran2,4, Elena De la Cruz1, Ricardo Nalda-Molina3,4, Isabel Gascón-Ros1, Amelia Ramón-López3,4.
1Department of nephrology, Hospital Dr Balmis Alicante, alicante, Spain; 2Pharmacy, Hospital Dr. Balmis Alicante, Alicante, Spain; 3 Division of Pharmacy and Pharmaceutics, School of Pharmacy, Miguel Hernández University, San Juan de Alicante, Spain; 4Alicante Institute for Health and Biomedical Research (ISABIAL), Alicante, Spain
Introduction: The initial dose of envarsus in renal transplant according to the manufacturers is 0.17mg/kg/ 24h. This recommended dose could be excesive in the real world scenario.
The objetive of this study is the evaluation of four different initial doses of envarsus in renal transplant recipients de novo.
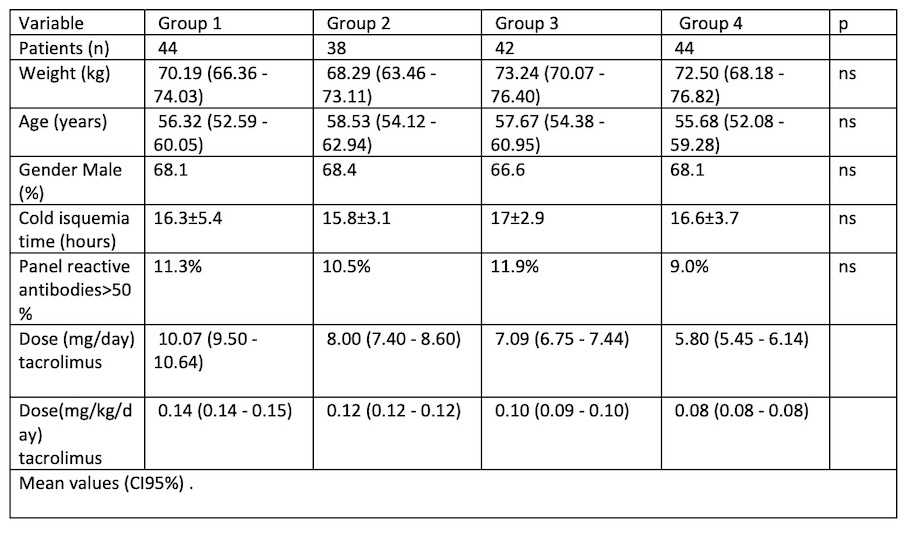
Material and methods: prospective observational study of four arms with adult renal transplant recipients from deceased donors receiving thymoglobulin, sirolimus, prednisone and four different doses of envarsus started when serum creatinine was under 3 mgr/dl as immunossuppesion. Group 1(G1): 0.15, Group 2(G2): 0.12, Group 3(G3): 0.1, Group 4(G4): 0.08mg/kg.
The pharmacokinetical data analyzed were the area under curve (AUC) obtained from 10 points of tacrolimus plasmatic concentrations (Cp) after 3 doses and trough Cp 72 hours (Cp_72h)) after starting the treatment. The therapeutical ragne (TR) considered were Cp 6-8 ng/mL (within range), Cp>8 ng/mL (overexposure) and Cp<6 ng/mL (underexposure). The follow up period was at less 3 months. Renal function was evaluated by glomerular filtration rate.
Homogenización variables were: recipient age and gender, weight, cold isquemia time and immunological risk. They are shown with tacrolimus dose in mg and mg/kg.
Comparative variables were: Trough Cp and AUC at 72 hours, recipients in each TI after 3 doses and percentage of pacients with omitted dose in case of over range
Results: A total of 168 pacients were enrolled(67.5% males), mean age 57 años (IC95%: 54,8-59,1) and mean weight 70,5 kg (IC95%: 68,2-72,3); G1: n=44, mean initial dose 10,1 mg/24h (IC95%:9,5-10,7) and 0,144 mg/kg/24h (IC95%: 0,141-0,146); G2: n=38, 8 mg/24h (IC95%:7,4-8,6) and 0,117 mg/kg/24h (IC95%: 0,115-0,119); G3: n=44 , mean initial dose 7 mg/24h (IC95%:6,6-7,4) and 0,096 mg/ kg/24h (IC95%: 0,094-0,099).
Mean Cp_72h and AUC in G1and G2 were similar, but significatively higher than in G3 and G4 (p:0,002). The percentage of patients with Cp_72h within therapeutic range was similar in all groups but the percentage with overexposure was statistically superior in G1 and G2 than in G3 and G4, being the percentage in G4 the lowest. 31,8% and 34,2% of recipients in G1 and G2 vs. 14,3% and 15,9% in G3 and G4, repectively, had to skip one dose due to a high trough Cp. The renal function was similar in the 4 groups at 3 months posttransplant.
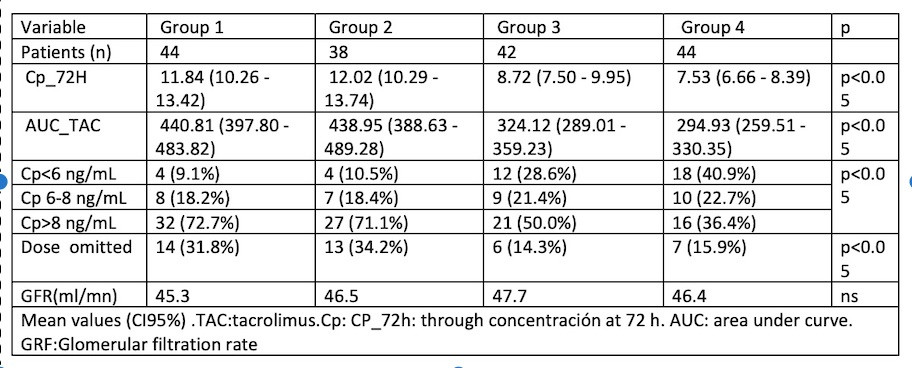
Conclusions: A dose of 0.08 mg/kg/24h, which is less than half of the recommended dose, achieves a suitable Cp and AUC. This results in a lower percentage of recipients experiencing overexposure trough Cp and omitted doses, while still achieving the same results and avoiding overdose.
[1] renal transplant
[2] envarsus
[3] induction
[4] overdose
[5] area under curve
