
Belatacept conversion in pancreas transplantation: Promising insights from a cohort study
Christophe Masset1, Claire Garandeau1, Simon Ville1, Magali Giral1, Aurélie Houzet1, Julien Branchereau1, Benoit Mesnard1, Gilles Blancho1, Jacques Dantal1, Diego Cantarovich1.
1Center for Research in Transplantation and Translational Immunology, UMR 1064, Institut de Transplantation Urologie Néphrologie , Nantes, France
The use of belatacept in maintenance therapy of kidney transplant recipients allows withdrawal of calcineurin inhibitors and thus improvement in allograft function. Despite being of interest in pancreas transplant recipients due to the additional relief on β-cell toxicity, data on the subject are scarce.
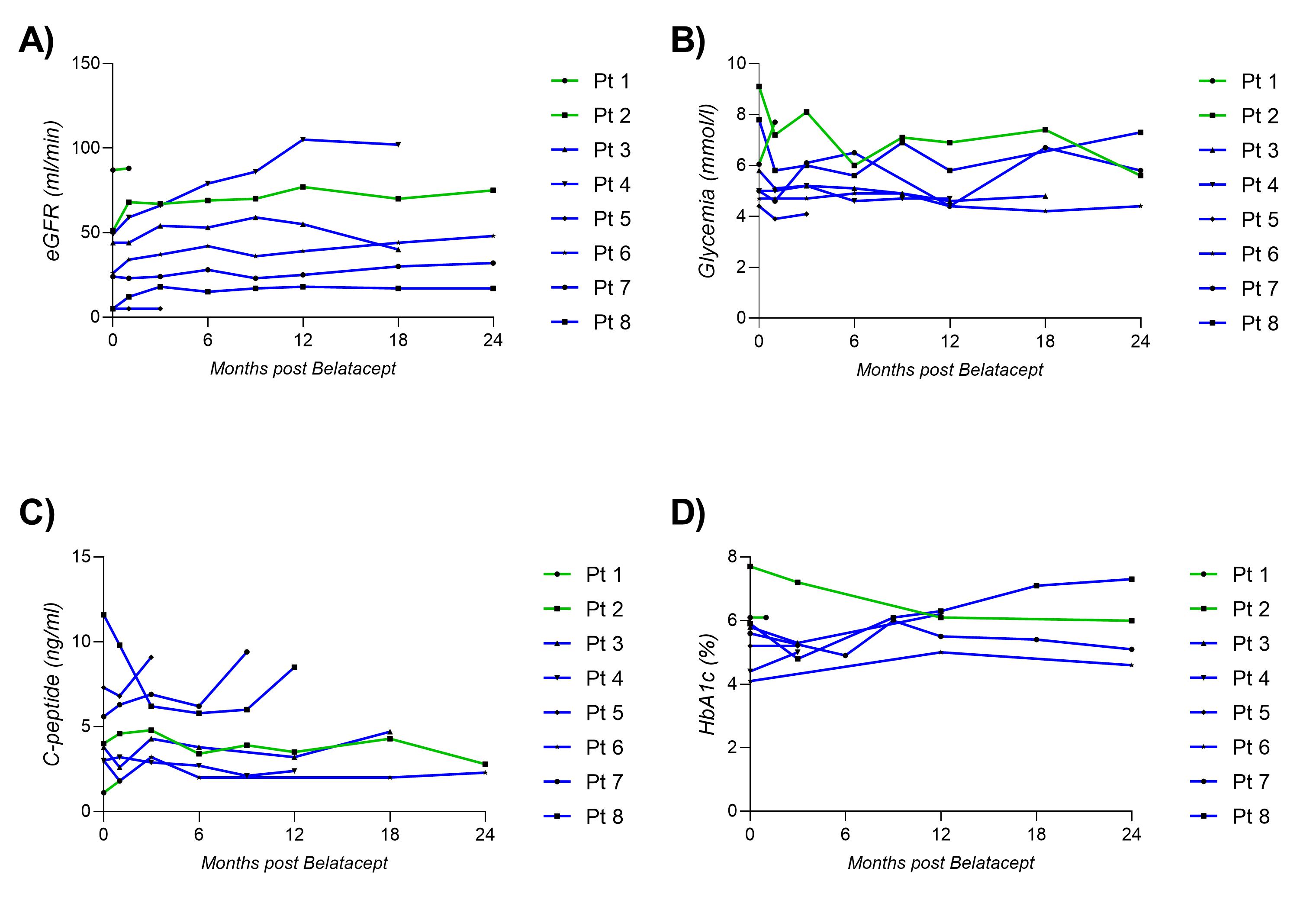
We report 8 pancreas transplant recipients (6 Simultaneous Pancreas Kidney and 2 Pancreas Transplant Alone) converted to belatacept due to calcineurin inhibitor toxicity (kidney n = 6, pancreas n = 2). One year after conversion, we observed significant improvement in kidney function (mean eGFR = +19ml/min) and β-cell function (HbA1c from 7.7% to 6%).
Importantly, no rejection episodes nor serious infectious complications were observed. Those observations suggests belatacept conversion as a valuable strategy in order to preserve kidney and pancreatic functions in pancreas transplant recipients. Whilst our results are promising, further studies are required to fully assess the role for belatacept conversion in pancreas transplantation.
[1] Pancreas transplantation
[2] Belatacept
[3] Beta cell toxicity
