Favourable outcomes of recipients of SARS-CoV-2 RNA positive donor organs support their safe utilisation in the post-pandemic era
Ines Ushiro - Lumb1, Suzie Phillips1, Christie Geoghegan1, Rhiannon Taylor1, Rommel Ravanan1, Derek Manas1, Douglas Thorburn1, Chris Callaghan1.
1NHS Blood and Transplant, London, United Kingdom
Introduction: Nucleic acid testing (NAT) for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA in upper and lower respiratory tract samples is a prerequisite for deceased organ donation in the UK. NAT positive donors with no Coronavirus disease 2019 (COVID-19) as a cause of death are assessed for suitability for organ donation. International short-term outcome data on recipients of SARS-CoV-2 NAT positive donor organs have so far indicated safety in utilisation of non-lung organs. We have analysed the cumulative data for UK transplants and hereby present a short summary with the main message deriving from this analysis.
Methods: SARS-CoV-2 RNA screening results for each potential deceased organ donor characterised in the UK is recorded by NHS Blood and Transplant. Laboratory proven SARS-CoV-2 infection in solid organ transplant recipients is captured by national registry linkage. Recipient outcome reports are received by NHSBT at regular intervals, starting at 30 days post-transplantation. Patients who received organs from donors who tested positive for SARS-CoV-2 RNA were compared to a control group whose donors had tested negative at screening, during the same period.
Results: Between March 2020 and June 2023, 6090 potential deceased donors were assessed; 161 tested SARS-CoV-2 NAT positive during donor characterisation, with 94 becoming actual donors. 250 organs from these donors were transplanted into 236 recipients (153 kidney-only, 14 simultaneous pancreas-kidney, 60 whole liver, 10 split liver, 2 pancreas-only, 11 heart, 3 bilateral lungs). Recipients were screened for SARS-CoV-2 pre- and post-transplant as per routine local protocol, with no demonstrable cases of donor-derived transmission of infection. After excluding some cases (e.g., paediatric and super urgent cases, cases with missing information), 51 adult liver recipients, and 153 adult kidney recipients, were compared to 1755 liver and 6936 kidney recipients in the control group, with no difference in outcome. Numerous other parameters were compared, including early post-transplant complications such as arterial and venous thrombosis and haemorrhage requiring re-operation, with no difference. Table 1 shows a subset of the parameters analysed, using follow up information available as of 3rd March 2024.
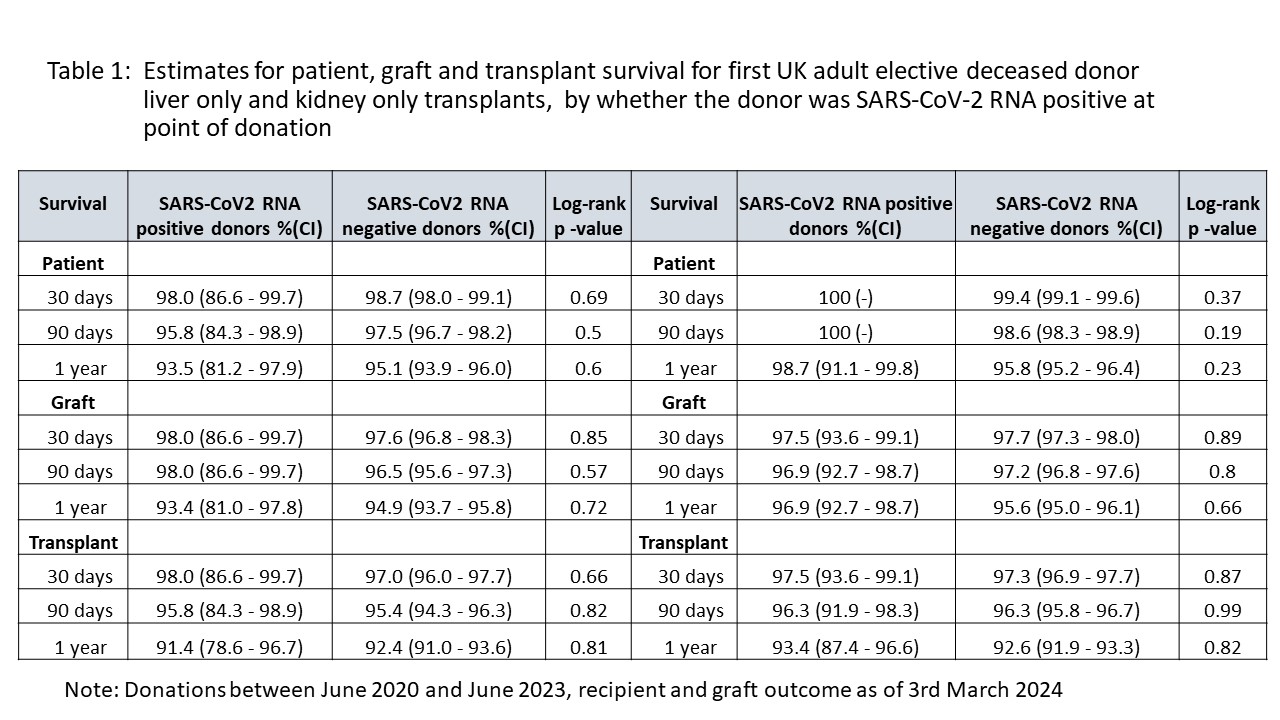
Conclusion: Our national experience to date indicates that organs other than lungs, from SARS-CoV-2 NAT positive deceased donors whose cause of death is not COVID-19, should be characterised and considered for transplantation in the same way as SARS-CoV-2 NAT screen negative donors. There is no evidence of graft-related transmission of infection. With follow-up beyond 1 to 2 years, available data does not indicate there is a cause for concern in terms of graft and recipient outcome. It is hoped that such data will be used to consolidate guidance and practice, increasing safe organ utilization from a larger pool of donors.
[1] SARS-CoV-2
[2] COVID-19
[3] Organ Utilization
[4] Deceased Organ Donor
[5] Recipient Outcome
[6] Coronavirus-19 Disease
