Multinational, retrospective study assessing outcomes and healthcare resource utilization in solid organ transplant recipients with cytomegalovirus infection and refractory, resistance, or intolerance to anti-cytomegalovirus treatments: analysis of impact of viremia clearance on outcomes
Kimberly Davis1, Luís Veloso2, Tien Bo1.
1Takeda Development Center Americas, Inc., Lexington, MA, United States; 2CTI Clinical Trial & Consulting Services, Lisbon, Portugal
Introduction: Cytomegalovirus (CMV) is a common infection among solid organ transplant (SOT) recipients, and can result in an increased risk of morbidity and mortality in these patients. This analysis of real-world data aimed to describe the impact of viremia clearance on clinical outcomes and healthcare resource utilization in SOT recipients with refractory or resistant CMV infection, or intolerance to anti-CMV agents.
Method: This multicenter, retrospective study included adult SOT recipients with refractory, resistant or intolerant (RRI) CMV infection. Data between 2014 and 2021 were collected from 13 centers across Europe and the USA. The analysis descriptively compared demographics, patient characteristics, clinical outcomes, and hospitalizations in patients who achieved viremia clearance during the first RRI CMV episode versus those in patients who did not achieve viremia clearance.
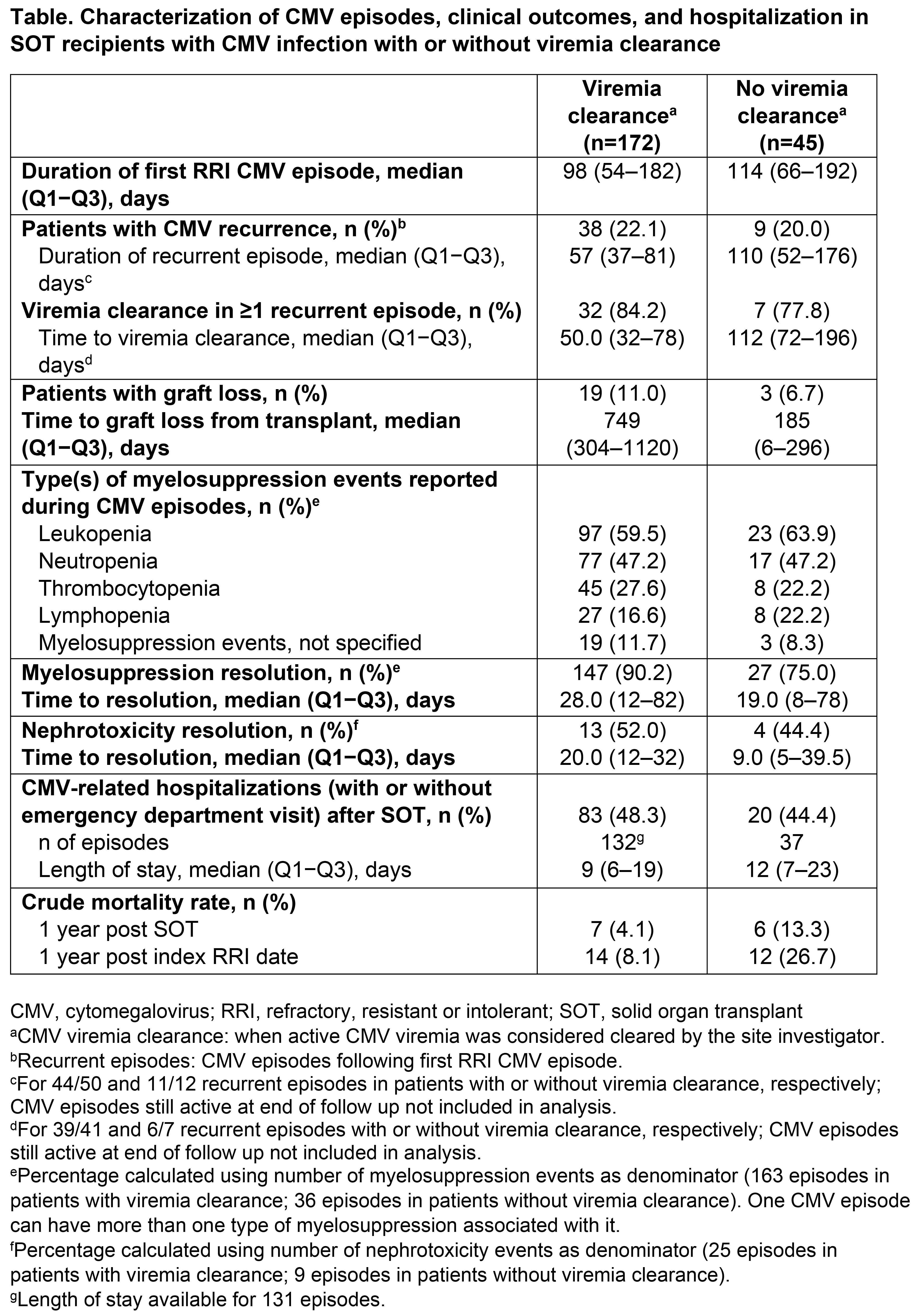
Results: Among 217 patients with a first RRI CMV episode and viremia clearance data, 172 (79%) achieved viremia clearance and 45 (21%) did not. In patients with vs without viremia clearance, respectively, median age was 58 vs 57 years, and 59% vs 82% were male. Of the 172 first RRI CMV episodes with viremia clearance, 54% were identified as refractory, 31% as intolerant, and 26% as resistant (categories not mutually exclusive). Of the 45 first RRI CMV episodes with no viremia clearance, 56% were identified as refractory, 42% as intolerant, and 27% as resistant. CMV recurrence occurred in 22% and 20% of patients with vs without viremia clearance, respectively (Table). Of the patients with vs without viremia clearance, respectively, 19/172 (11%) vs 3/45 (7%) patients experienced graft loss following SOT (median time to graft loss: 749 vs 185 days, respectively; Table). Myelosuppression events during CMV episodes were reported in 98/172 (57%) and 24/45 (53%) patients with vs without viremia clearance, respectively (Table), with valganciclovir and ganciclovir the most often used at myelosuppression diagnosis. Nephrotoxicity events occurred in 19/172 (11%) and 8/45 (18%) patients with vs without viremia clearance, respectively (Table), and the agents most often used when nephrotoxicity events occurred were foscarnet followed by valganciclovir, vs valganciclovir followed by foscarnet, respectively. CMV-related hospitalizations with/without emergency department visits occurred in 48% of patients with viremia clearance vs 44% of patients without viremia clearance (Table). Notably, the overall mortality within 1 year of RRI identification in patients without viremia clearance was higher than in those with viremia clearance (27% vs 8%, respectively).
Conclusion: These results highlight the high disease burden in SOT recipients with RRI CMV infection. In this difficult-to-treat population, the mortality rate was higher in patients without viremia clearance than in patients with viremia clearance, and there was a trend towards earlier graft loss in patients without viremia clearance.
The study was funded by Takeda Development Center Americas, Inc., Lexington, MA, USA. Medical writing support for this abstract was provided by Joanne Vaughan, employee of Excel Scientific Solutions (Fairfield, CT, USA), and was funded by Takeda Development Center Americas, Inc., Lexington, MA, USA.
[1] Mortality
[2] Cytomegalovirus
[3] Viremia clearance
[4] Refractory or resistant cytomegalovirus
[5] Solid organ transplant
