Multinational, retrospective study assessing treatment patterns, outcomes, and healthcare resource utilization in solid organ transplant recipients with cytomegalovirus infection and intolerance to anti-cytomegalovirus treatments
Emily Blumberg1, Jennifer Chow2, Oscar Len3, Oliver Witzke4, Luís Veloso5, Jessica Auciello6, Kimberly Davis6.
1University of Pennyslvania, Philadelphia, PA, United States; 2Tufts Medical Center, Boston, MA, United States; 3Vall d’Hebron University Hospital, Barcelona, Spain; 4University Hospital Essen, Essen, Germany; 5CTI Clinical Trial & Consulting Services, Lisbon, Portugal; 6Takeda Development Center Americas, Inc., Lexington, MA, United States
Introduction: Cytomegalovirus (CMV), a common infection among solid organ transplant (SOT) recipients, can lead to a higher risk of mortality and morbidity in these patients. However, management of CMV infection can be limited by toxicities or intolerances with anti-CMV agents including ganciclovir, valganciclovir or foscarnet. This study aimed to describe treatment patterns, clinical outcomes, and healthcare resource utilization in SOT recipients with CMV infection and intolerance to anti-CMV agents based on real-world data.
Method: This multicenter, retrospective study included adult SOT recipients with CMV infection refractory or resistant to treatment, or with intolerance (RRI) to anti-CMV agents. Data were collected from 13 centers across Europe and USA between 2014 and 2021. This analysis focused on patients whose first CMV episode was considered intolerant to anti-CMV treatment; patients whose first CMV episode was considered resistant or refractory to anti-CMV treatment were excluded. Patients may have experienced previous non-RRI episodes and/or subsequent CMV episodes (including RRI) after their first intolerant episode. Treatment patterns, outcomes, and hospitalizations were analyzed using descriptive statistics.
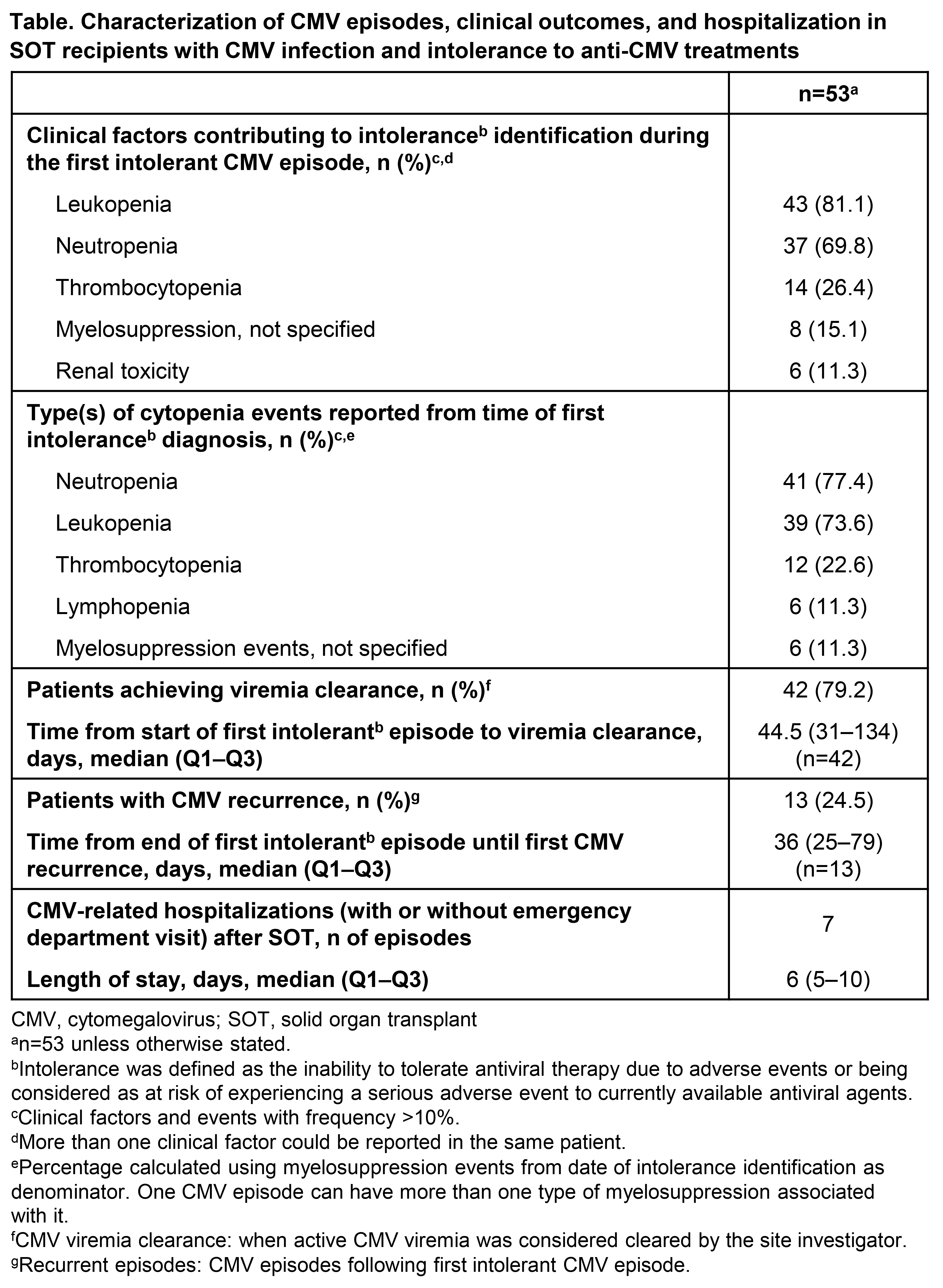
Results: In total, 53 of 218 patients enrolled were intolerant to anti-CMV agents (median age: 56 years; 72% male). Median (Q1–Q3) time from SOT until first intolerance identification was 211 (128–265) days. Patients spent a median (Q1–Q3) of 21 (8–30) days on anti-CMV treatment before intolerance identification. At intolerance identification, and for all CMV episodes, valganciclovir was the most frequently used anti-CMV agent (81% and 87% respectively), followed by ganciclovir (13% and 32%; patients could receive ≥1 type of anti-CMV agent during a CMV episode). Of 51 patients with available data, 15 (29%) received ≥2 therapies for all CMV episodes. One or more anti-CMV therapy dose change was prescribed for 91% of patients, most commonly with valganciclovir (83%) or ganciclovir (35%). Reasons for dose changes with valganciclovir included CMV viremia resolution (55%), lower dose for maintenance (35%), and leukopenia (25%). Intolerance was most commonly due to myelosuppression or renal toxicity (Table). Death due to any cause was 28% (median follow-up 1295 days) and mortality at 1-year post-intolerance identification was 17%. Viremia clearance was achieved in 42 (79%) patients during their first intolerant CMV episode (Table). Seven (13%) patients experienced 1 CMV-related hospitalization, and ten (19%) patients experienced organ rejection.
Conclusion: These results highlight the high burden of intolerance to anti-CMV agents in SOT recipients with CMV infection. Alongside the risk of adverse outcomes, many patients fail to achieve viremia clearance and/or experience recurrence. There is a need for treatments with improved safety profiles that can achieve and maintain CMV clearance.
This study was funded by Takeda Development Center Americas, Inc., Lexington, MA, USA. Medical writing support for this abstract was provided by Emma Green, PhD, employee of Excel Scientific Solutions (Fairfield, CT, USA), and was funded by Takeda Development Center Americas, Inc., Lexington, MA, USA.
[1] Mortality
[2] Cytomegalovirus
[3] Antiviral therapy
[4] Maribavir
[5] Refractory
[6] Resistant
[7] Intolerant
