
Optimisation of donation after circulatory death livers – removal of extracellular histones during normothermic perfusion and following whole blood reperfusion in an ex-situ porcine model
Syed Hussain Abbas1, Fungai Dengu1, Jeremy Schofield2, Guozheng Wang2, Alireza Morovat3, Alberto Quaglia4, Simon T Abrams2, Cheng-Hock Toh2, Andrew Aswani5, Peter Friend1.
1Nuffield Department of Surgical Sciences, University of Oxford, Oxford, United Kingdom; 2Department of Clinical Infection, Microbiology and Immunology, University of Liverpool, Oxford, United Kingdom; 3Department of Biochemistry, John Radcliffe Hospital, Oxford, United Kingdom; 4Department of Histopathology, Royal Free Hospital, London, United Kingdom; 5Santersus, AG, Zurich, Switzerland
Introduction: Donation after circulatory death (DCD) livers are at risk of primary non-function (PNF) and intra-hepatic-biliary stricture formation due to increased susceptibility to ischaemia reperfusion injury (IRI). This study aims to assess the impact damage associated molecular pattern (DAMP) removal (in particular, extracellular histones) during normothermic machine perfusion (NMP) and following whole blood reperfusion (ex-situ simulated transplant model) using porcine DCD livers.
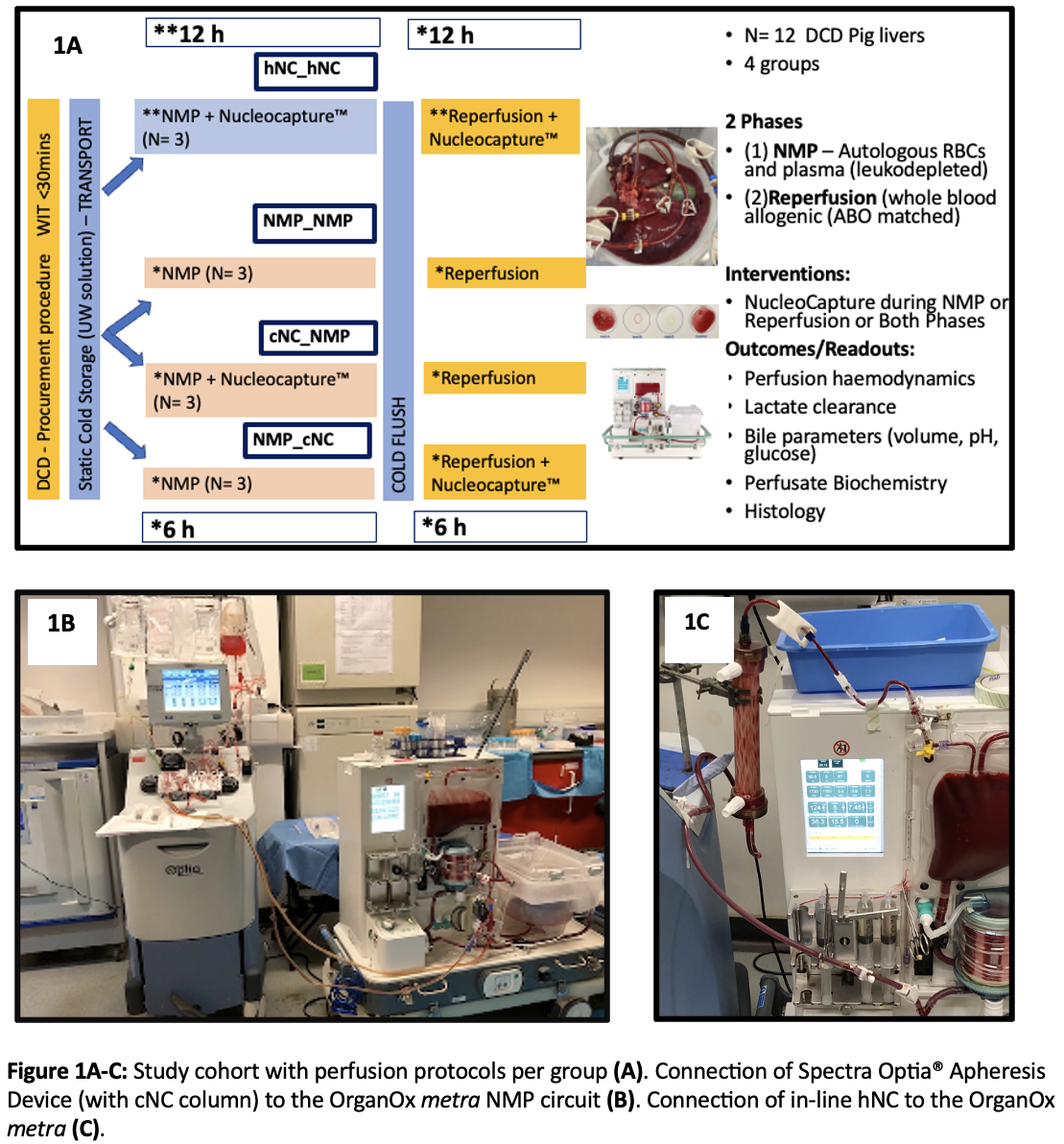
Methods: A total of 12 DCD porcine livers were included in this study (Figure1A). Initial perfusions involved circulating DAMP removal using the classic NucleoCapture column (cNC), connected to the OrganOx metra NMP circuit via a Spectra Optia® Apheresis device (Figure1B). Perfusions consisted of a 6h NMP phase using leukodepleted blood and a 6h reperfusion phase using whole blood. NucleoCapture was either applied in the NMP phase (cNC-NMP: n=3) or in the reperfusion phase (NMP-cNC: n=3) and compared to standard continuous NMP in both phases (NMP-NMP: n=3). Subsequent experiments were extended to 12h in both perfusion and reperfusion phases with application of a continuous in-line NucleoCapture hemoperfusion column prototype (hNC-hNC: n=3) (Figure1C). Lactate clearance, hepatocellular injury and extracellular histone levels were compared between groups using repeated measures 2-way ANOVA.
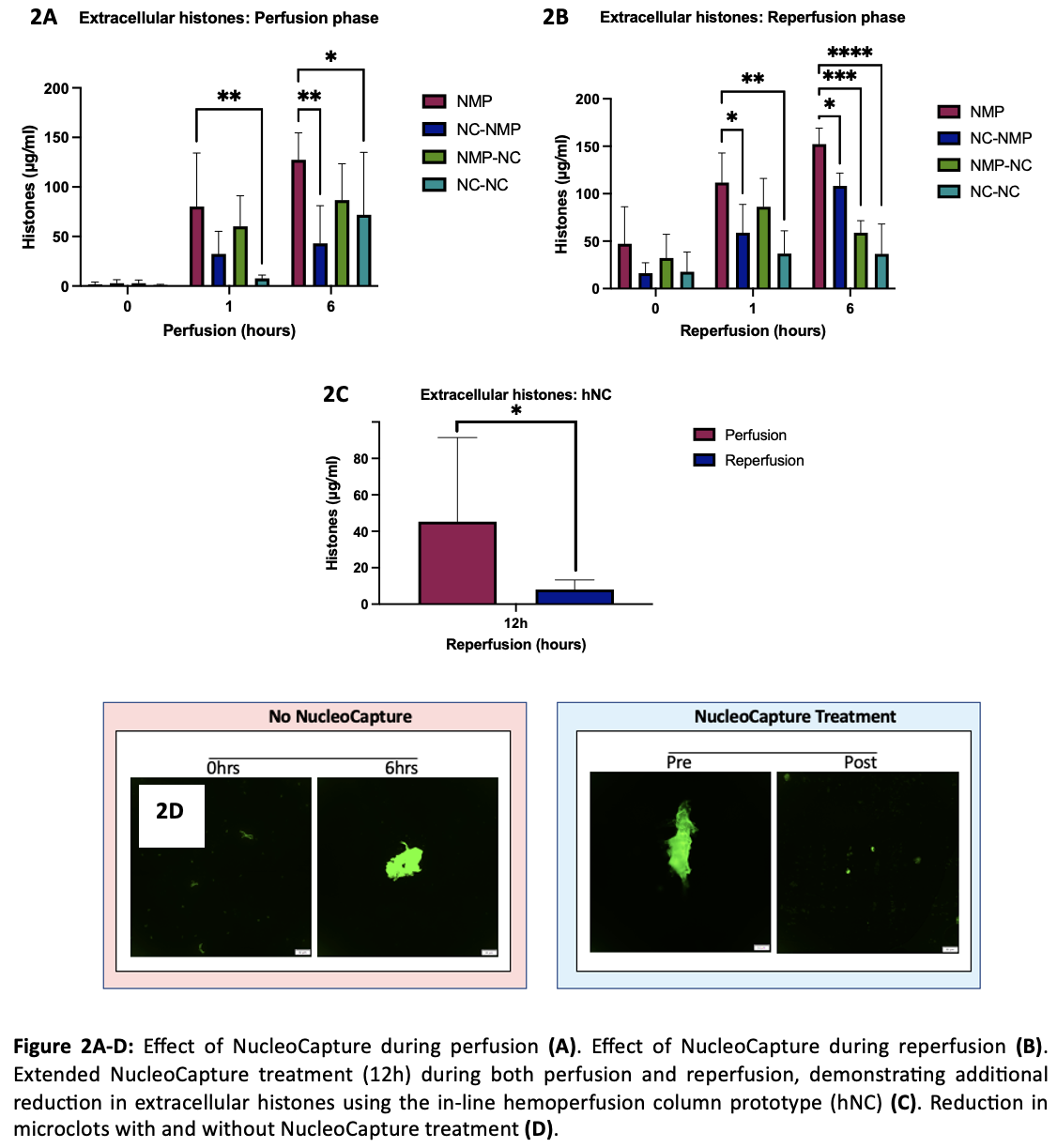
Results: NucleoCapture significantly reduced circulating extracellular histones using the cNC (P=0.003) and hNC (P=0.041) column compared to standard NMP at 6h perfusion (Figure2A). This effect was also observed during reperfusion using cNC (P=0.0001) and hNC (P<0.0001) at 6h reperfusion (Figure2B). There was a significant reduction in extracellular histones at the end of 12h reperfusion (8ug/ml±5.4) compared to 12h perfusion (45ug/ml±46.2) using hNC (Figure2C). Overall, clearance of histones resulted in improved haemodynamics, lactate clearance and reduction in microclots (Figure2D).
Discussion: NucleoCapture effectively removes circulating DAMPS from the perfusate demonstrating benefit in both ex-situ perfusion and reperfusion. If translated into clinical practice, this technology may improve outcomes of DCD liver transplantation.
[1] NMP
[2] Liver perfusion
[3] DCD
[4] Histones
[5] NETs
[6] Nucleosomes
