
The role of hypoxia inducible factor modulation and defatting in discarded steatotic human livers preserved with normothermic machine perfusion (NMP): observations from an ex-situ whole blood transplant model
Syed Hussain Abbas1, Sadr Shaheed1, Alberto Quaglia2, Alireza Morovat3, Fungai Dengu1, Georg Ebeling1, Alex Sagar1, Carlo Ceresa1, Leanne Hodson4, Christopher Pugh4, Peter Friend1.
1Nuffield Department of Surgical Sciences, University of Oxford, Oxford, United Kingdom; 2Department of Histopathology, Royal Free Hospital, London, United Kingdom; 3Department of Biochemistry, John Radcliffe Hospital, Oxford, United Kingdom; 4Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom
Introduction: The hypoxia-inducible factor (HIF) pathway has been implicated in hepatic steatosis with the HIF-1a isoform providing a hepatoprotective effect through enhancement of liver fat metabolism and the HIF-2a isoform conversely promoting intrahepatic lipid accumulation. Pharmacological stabilisation of HIF-1a of steatotic livers during normothermic machine perfusion (NMP) may enable a greater proportion of livers to achieve ex-situ functional criteria for transplantation. We have previously reported dosing schedules for pharmacological HIF modulators including deferoxamine (DFO, a potent activator of both HIF-1a & HIF-2a) with selective HIF-2a inhibition using PT2385 (a HIF-2a dimerisation inhibitor) during NMP. The aim of the present study was to compare the effect of an established NMP defatting protocol (DeFat, comprising of insulin/glucose reduction, L-carnitine, NKH477 and lipid filter) with and without the adjunct of HIF modulators (DeFat-HIF, addition of DFO & PT2385) during ex-situ perfusion.
Methods: Six sequential discarded livers (DeFat, n=3 and DeFat-HIF, n =3) were perfused with pRBC using the OrganOx metra over 12h, flushed and reperfused for 6h with whole blood (ex-situ transplant model), (Table1). Global hepatocellular function was measured using the LiMAx test. Macrosteatosis (MaS) was quantified using Visiopharm® digital image analysis software and blinded histopathologist report. HIF activation was determined through serial perfusate EPO measurements.
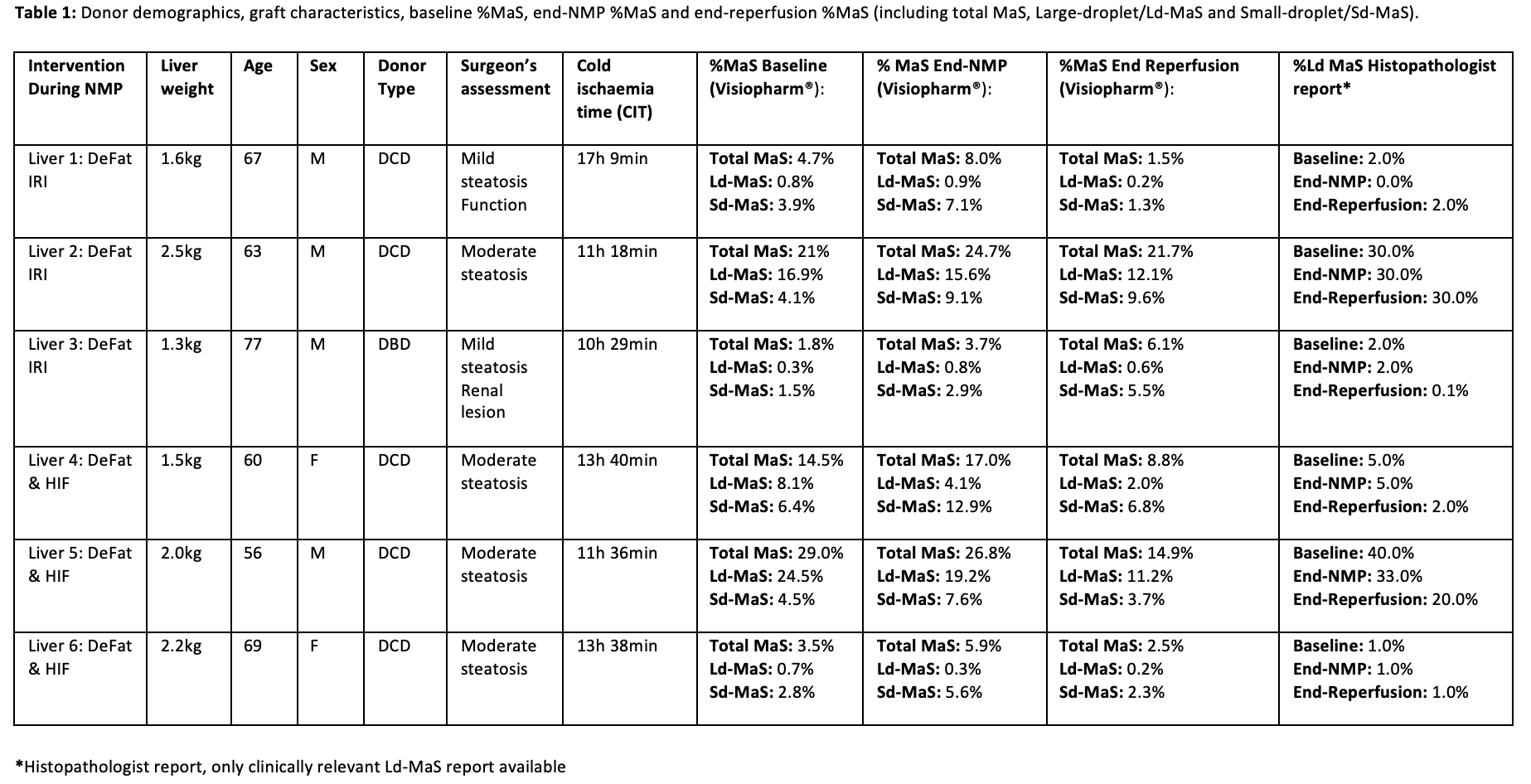
Results: Both groups demonstrated comparable lactate, pH, ALT and LiMAx test results with the exception of triglyceride/cholesterol mobilisation and glucose metabolism which was greater in the DeFat-HIF group (Figure1). The DeFat-HIF group had significantly higher levels of perfusate EPO following 12h of perfusion (233.7±107.2 miU/ml vs. 23.4±12.7 miU/ml, P = 0.03) and first 2h of reperfusion. A pronouced reduction in Ld-MaS was only observed in the DeFat-HIF group (Table1).
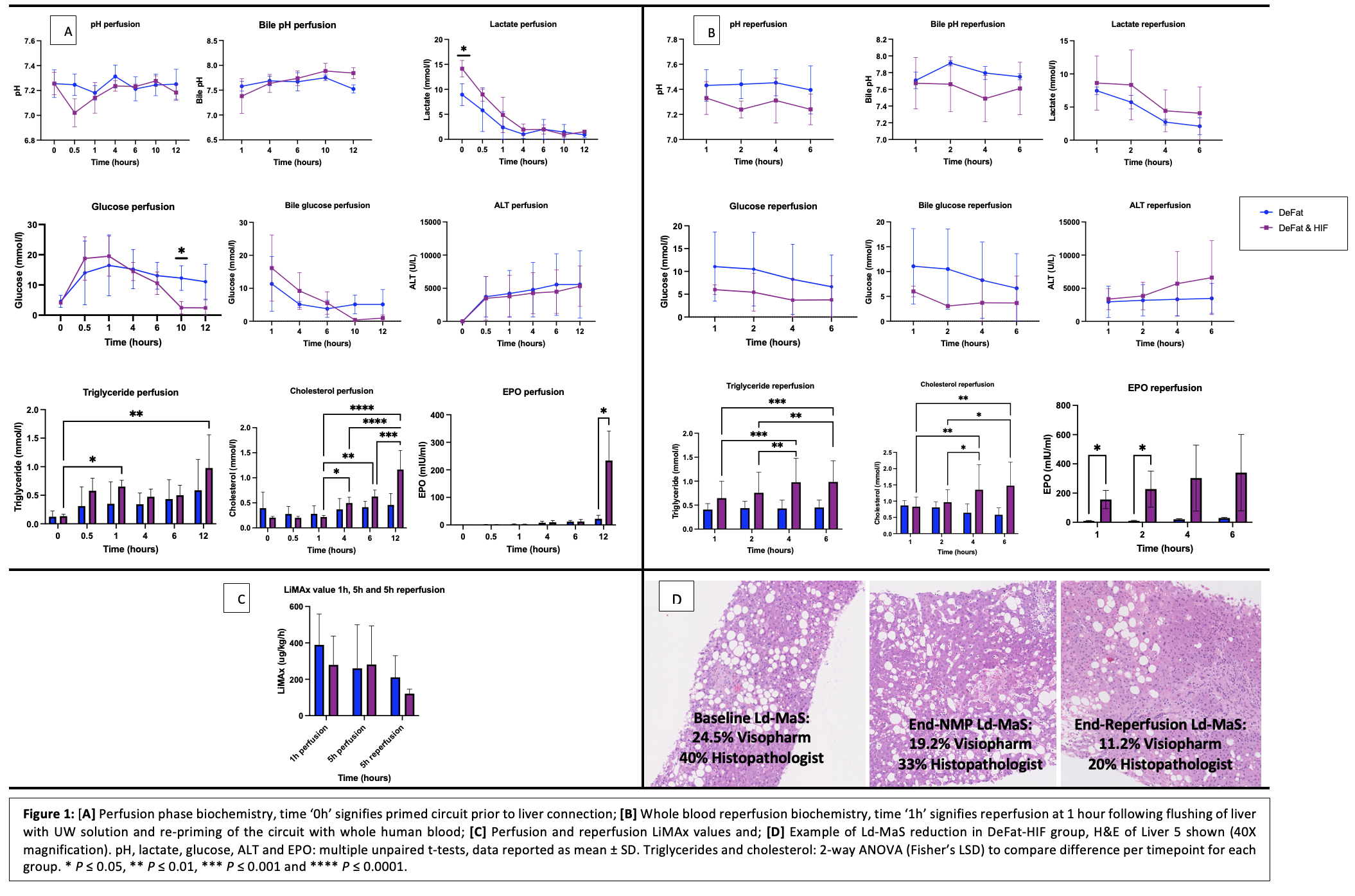
Conclusion: The DeFat-HIF protocol demonstrated a decrease in the presence of Ld-MaS and comparable perfusion biochemistry to the established DeFat protocol. The effect of these interventions on ischaemia-reperfusion injury remain to be elucidated.
