
Detection of kidney allograft rejection using urinary chemokines: A prospective multicenter cohort study (KTD-Innov)
Valentin Goutaudier1,2, Maud Racapé1, Olivier Aubert1,2, Marta Sablik1, KTD-Innov Investigators3, Pierre-Antoine Gourraud4, Carmen Lefaucheur1,5, Sophie Brouard4, Dany Anglicheau1,2, Alexandre Loupy1,2.
1Paris Institute for Transplantation and Organ Regeneration, INSERM U970, Paris, France; 2Necker Hospital, AP-HP, Paris, France; 3KTD-Innov Consortium, National Research Agency, Paris, France; 4Nantes University Hospital, Nantes University, Nantes, France; 5Saint-Louis Hospital, AP-HP, Paris, France
KTD-Innov Consortium.
Introduction: Urinary chemokines C-X-C motif ligands 9 (CXCL9) and 10 (CXCL10) have shown promise in models for detecting kidney allograft rejection, but require validation in specifically designed prospective multicenter studies. The KTD-Innov study (ClinicalTrials.gov, NCT03582436) aimed to confirm their clinical utility in a large, prospective, multicentric, and unselected cohort of kidney transplant patients.
Method: We enrolled consecutive adult patients who underwent a kidney transplantation in 7 transplant referral centers between July 2018 and December 2019. We prospectively quantified urinary CXCL9 and CXCL10 protein levels using an automated immunoassay platform. The primary outcome was allograft rejection (AMR, TCMR, mixed) within the first year post-transplantation, assessed according to the international Banff 2019 classification. We evaluated if urinary chemokine levels provided additional value beyond standard of care (SOC) parameters in predicting allograft rejection, and externally validated the performances of existing chemokine-based models.
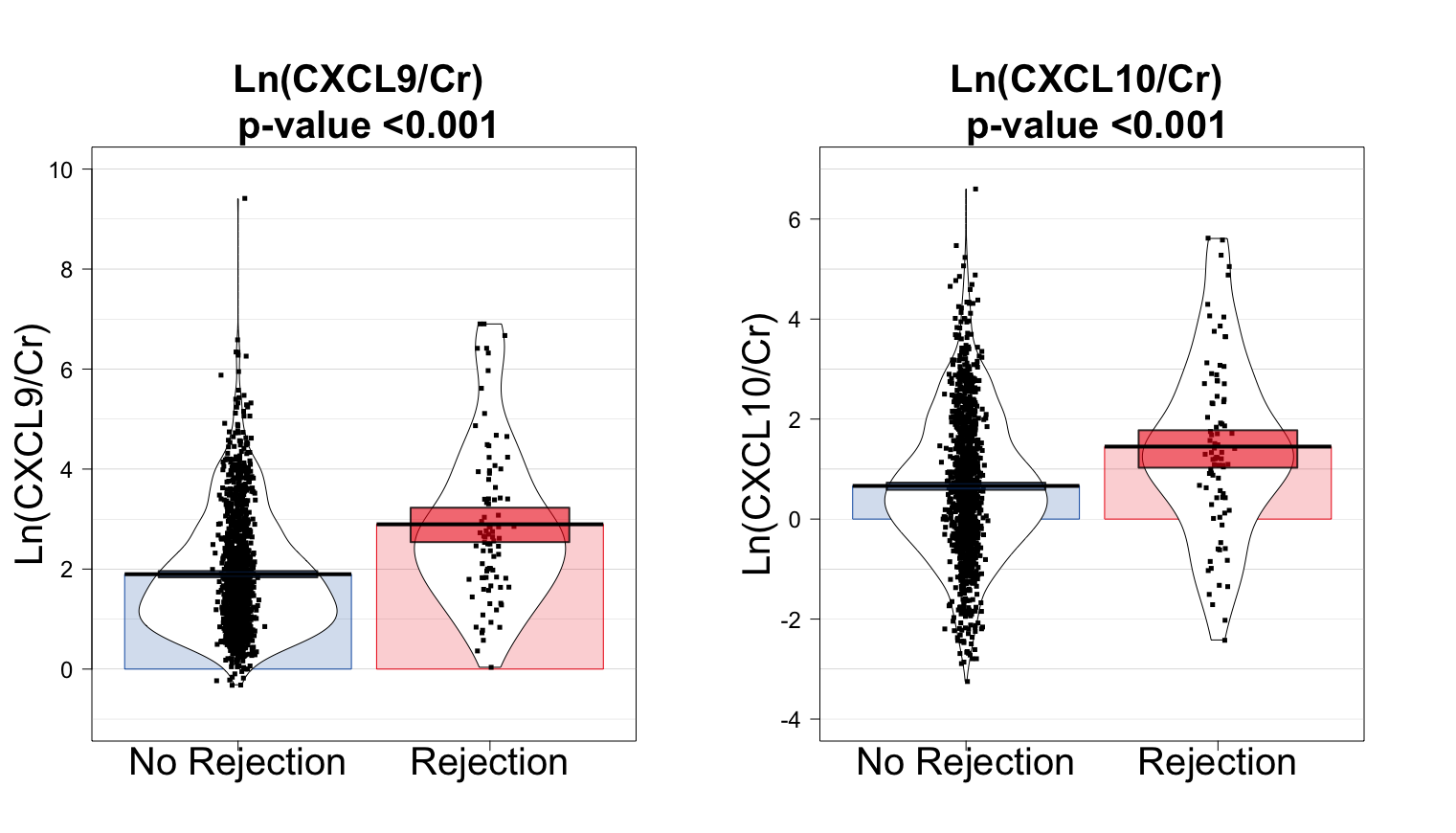
Results: Overall, 733 patients were included in the main analysis, with 1,549 biopsies paired with a urine sample. The median follow-up after transplantation was 12.3 months (interquartile range, 11.9-13.1 months). The cumulative incidence of rejection per patient was 9.7% (95% confidence interval [CI], 7.6%-12.1%). Urinary CXCL9 and CXCL10 levels were significantly associated with allograft rejection (p < 0.001 for both, Figure 1).
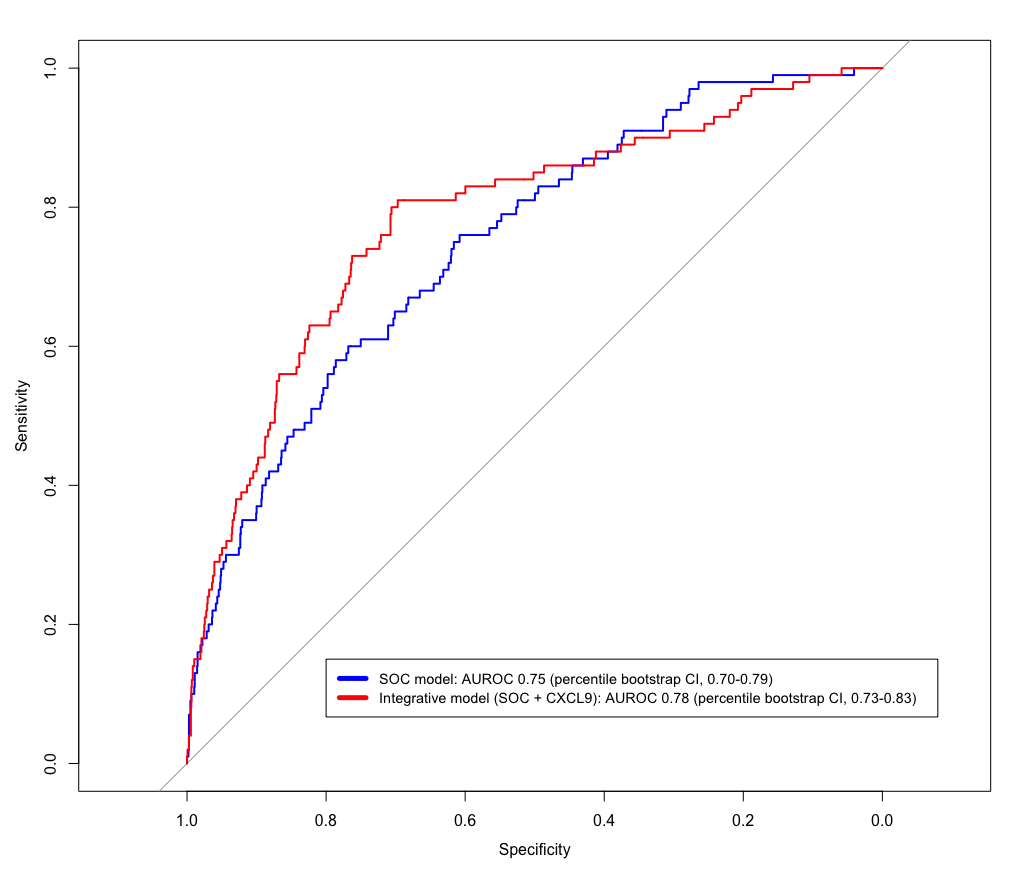
Urinary CXCL9 levels, but not urinary CXCL10 levels, showed an additional value beyond SOC parameters for predicting allograft rejection (area under the receiver operating characteristic curve [AUROC] of the SOC + CXCL9 model = 0.78, percentile bootstrap CI 0.73-0.83; AUROC of the SOC model = 0.75, percentile bootstrap CI 0.70-0.79; Figure 2).
Integrating urinary CXCL9 levels with SOC parameters allowed to avoid 6 biopsies per 100 patients when the risk for allograft rejection was predicted at 10%. In the KTD-Innov cohort, existing chemokine-based models demonstrated low to moderate performances to predict allograft rejection, with no significant additional clinical value of urinary chemokine levels.
Conclusion: In this large, prospective, and unselected cohort study, urinary chemokine levels showed moderate clinical benefit in detecting kidney allograft rejection within the first year after transplantation.
The KTD-Innov study was funded by the French government, with financial support managed by the National Research Agency (ANR) under the program “Investissements d’avenir”, with the grant agreement no. ANR-17-RHUS-0010.
[1] Kidney transplantation
[2] Multicentric cohort
[3] Allograft rejection
[4] Biomarkers
[5] Urinary chemokines
[6] Monitoring
