Comparative profiles of circulating T-cell subsets and cytokines in Kidney allograft recipients with inflammatory interstitial fibrosis and tubular atrophy and Chronic antibody-mediated rejection
Brijesh Yadav1, Narayan Prasad1, Vikas Agarwal2, Vinita Agrawal3, Manoj Jain3.
1Nephrology and Renal Transplantation, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India; 2Clinical Immunology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India; 3Pathology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India
Narayan Prasad.
Introduction: Cytokines are small protein molecules that modulate both the cellular and humoral immune response against the transplanted allograft and thus may modulate renal allograft outcome. The cytokine milieu induces specific T cell lineage commitment, signaling mediator activation, chemokine expression in endothelial cells, and the recruitment of mononuclear cells in intragraft tissue. Thus, a predominance of specific T cell subsets and their cytokines may determine the specific nature of the immune response and, consequently, the nature of allograft rejection and graft outcome. There is a dearth of data on circulating T cell subsets and cytokines profiles in renal transplant recipients (RTRs) with inflammatory interstitial fibrosis and tubular atrophy (i-IFTA) and Chronic antibody-mediated rejection (CABMR). Therefore, in this study, we aimed to determine the circulating T-cell subsets specific transcription factors and different cytokines profiles in RTRs with stable graft function (SGF), Inflammatory interstitial fibrosis and tubular atrophy, and chronic antibody-mediated rejection.
Methods: In this study, RTRs with biopsy-proven (Banff 2015 diagnostic criteria) stable graft function (n=16), Chronic antibody-mediated rejection (n=42) and inflammatory interstitial fibrosis and tubular atrophy (i-IFTA) (n=32) were recruited. Stable graft function was defined as stable serum creatinine within 6 months, without significant proteinuria and <10% cortical surface area showing evidence of histological lesions. A 5ml blood sample in plain vials and 2ml in Trizole solution were collected. RNA was isolated from the Blood in Trizole solution . T cell subsets specific transcription factor (TF) was determined by the Taqman real-time PCR using GAPDH as housekeeping control (assay id: FAM_MGB_HS99999905_m1) T-bet (Th1 cell, assay id: FAM_MGB_HS00203436_m1), GATA-3 (Th2 cell, assay id: FAM_MGB_HS00231122_m1), RORC (Th17cell, assay id: FAM_MGB_HS01076122_m1), FoxP3 (Treg cell, assay ID: FAM_MGB_HS01085834_m1). Fold change was calculated by 2-(ΔΔCt) method relative to SGF. Serum was separated from plain vials of blood, and serum cytokines were analyzed by the ELISA. Data were analyzed with SPSS software using ANOVA and Bonferroni post-hoc tests, and the mean was compared.
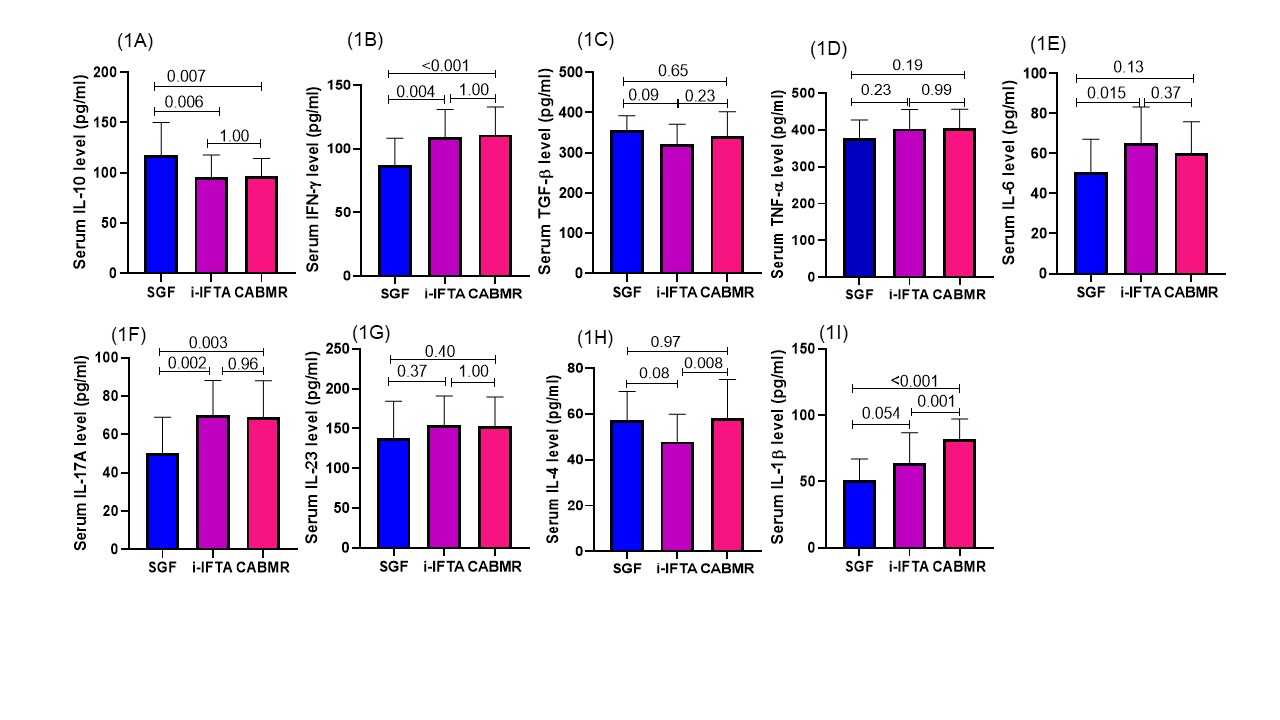
Results: The fold change in Th1 cell transcription factor T-bet expression in SGF vs CABMR group was (1.17±0.59 vs 2.84±1.34au; p<0.001), SGF vs i-IFTA was (1.17±0.59 vs 2.22±1.02 au; p=0.009). The fold expression of Th2 cell TF GATA-3 in the SGF vs CABMR group was (1.05±0.14 vs 1.18±0.82au; p=1.0), SGF vs i-IFTA was (1.05±0.14 vs 1.65±0.62au; p=0.016). The fold expression of Th17 cell TF RORC in SGF vs CABMR was (1.14±0.31 vs 2.33±0.95au; p<0.001), SGF vs i-IFTA (1.14±0.31 vs 2.00±0.70au; p=0.002) and regulatory T cell transcription FoxP3 expression in SGF vs CABMR was (1.01±0.065 vs 1.86±0.85au; p=0.002), SGF vs i-IFTA was (1.01±0.065 vs 2.26±0.91au; p<0.001). The ratio of T-bet/FoxP3, GATA-3/FoxP3 and RORC/FoxP3 TF expression in SGF vs CABMR and i-IFTA group was similar and statistically not significant among the group. However, in i-IFTA vs CABMR, the ratio of T-bet/FoxP3 (1.13±0.71 vs 1.79±1.19, p=0.011), and RORC/FoxP3 (1.01±0.49 vs 1.59±1.07; p=0.01) was significantly higher in CABMR group. The absolute serum level of cytokines IFN-ɣ, IL-17 and IL-1β was significantly higher in the CABMR and i-IFTA group compared to SGF {{AbstractFigure.1a-i}}. Whereas, the ration of IFN-ɣ/IL-10 in SGF vs i-IFTA vs CABMR (0.83±0.37 vs 1.20±0.35 vs 1.19±0.38; p<0.05), IL-17A/IL-10 (0.50±0.31 vs 0.71±0.25 vs 0.65±0.22; p<0.05), IL-1β/IL-10 (0.49±0.27 vs 0.70±0.26 vs 0.88±0.23; p<0.05) was significantly higher in i-IFTA and CABMR group respectively.
Conclusions: A higher Th1/Treg, Th17/Treg cell ratio and their effector cytokines (IFN-γ, IL-17) are associated with the pathogenesis of CABMR and i-IFTA in RTRs.
[1] Chronic antibody mediated rejection, i-IFTA, SGF, Cytokines
