Donor-derived cell-free DNA (dd-cfDNA) kinetics during rejection and quiescence after simultaneous pancreas and kidney transplant
Abraham Velazquez1, Shree Patel2, Jijiao Zeng2, Oyedolamu Olaitan1.
1Department of Surgery, Rush University System for Health, Chicago, IL, United States; 2CareDx, Inc, Brisbane, CA, United States
Introduction: Early detection and treatment of rejection after simultaneous pancreas and kidney transplant (SPKTx) is imperative to optimize outcomes. To date, serial monitoring of dd-cfDNA has shown to discriminate rejection after SPKTx, but a paucity of data describes the timeline and trajectory of dd-cfDNA changes during rejection. We describe for the first time, dd-cfDNA trajectories before & after rejection diagnosis, and within a quiescent reference cohort after SPKTx.
Method: This is a prospective, single-center study of SPKTx recipients enrolled from Nov 2018 to May 2021 that underwent serial dd-cfDNA (AlloSure®) testing at 2 weeks post SPKTx, followed by monthly for year 1, then every 3 months in years 2-3, and every 6 months thereafter. Rejection episodes included biopsy proven rejection (BR) and clinically diagnosed rejection (CR). CR was defined as a constellation of sustained elevations in dd-cfDNA, rising or dnDSA, or persistent elevations of lipase or creatinine. CR was treated presumptively. Baseline dd-cfDNA was defined as the patient specific nadir result obtained prior to rejection diagnosis. Month 0 was the date of rejection diagnosis. The reference quiescent cohort was defined as not meeting criteria for CR, BR, or other injury on biopsy during study.
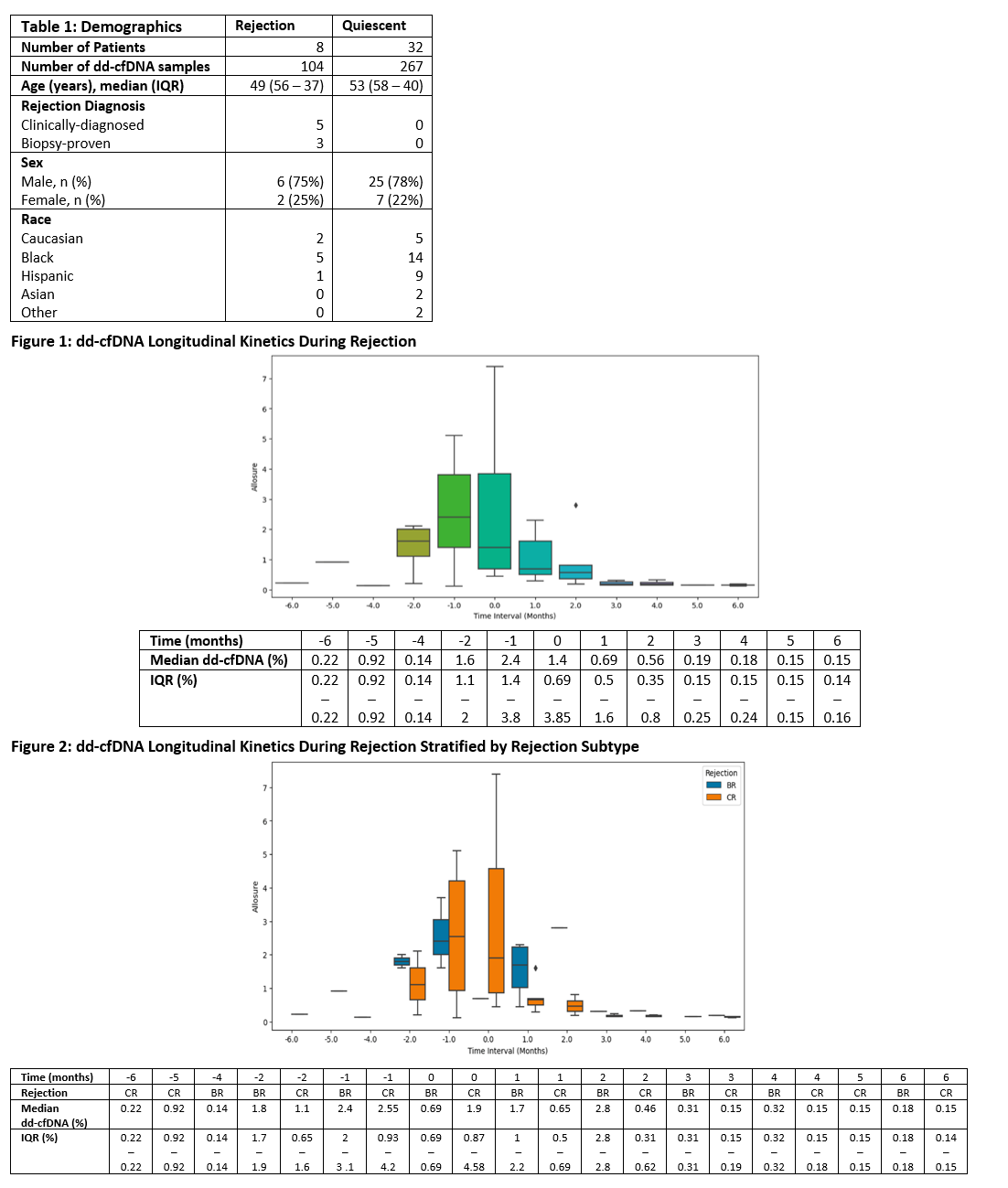
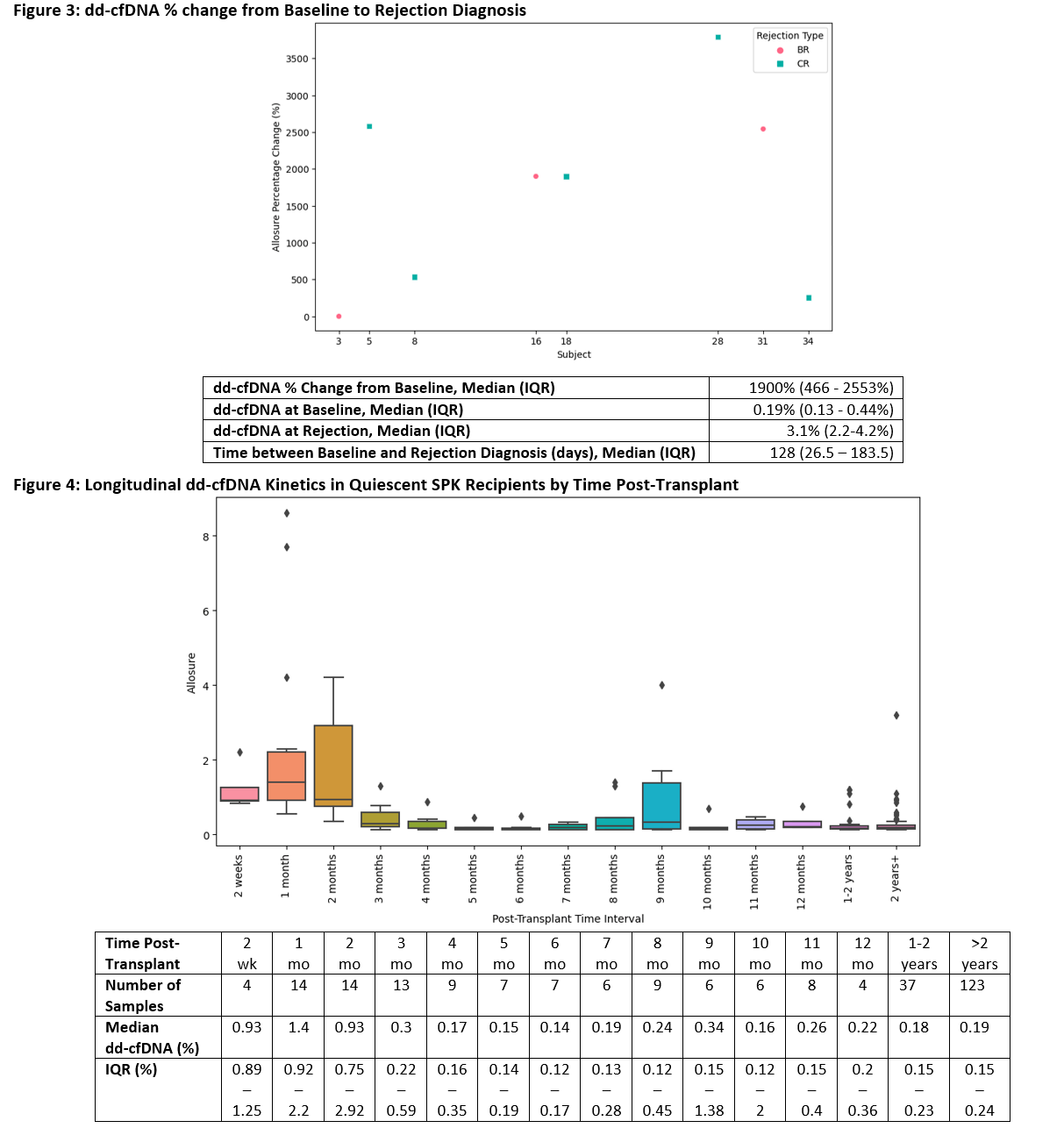
Results: 371 dd-cfDNA draws from 40 SPKTx recipients were analyzed. Table 1 outlines demographics (CR = 5, BR = 3, Quiescent = 32). Rejections were diagnosed at a median of 50 months (IQR 25 - 60 months) post SPKTx. Median dd-cfDNA values rose ahead of rejection diagnosis: 0.14% at -4 months, 1.6% at -2 months, and 2.4% at -1 month [Figure 1]. At time of rejection diagnosis (month 0), median dd-cfDNA was 1.4% (IQR 0.69-3.85%). Following diagnosis and treatment, median dd-cfDNA values fell: 0.69% at 1 month, 0.56% at 2 months, and 0.19% at 3 months. Similar trajectories were observed with CR and BR analyzed separately [Figure 2]. Median change in dd-cfDNA from baseline to rejection was 1900% (IQR 466 - 2553%) [Figure 3]. In the quiescent cohort, median dd-cfDNA levels were elevated early post SPKTx (2 weeks - 2 months) but followed a downward trajectory until reaching normal levels by 3 months and then remained relatively stable out to >2 years [Figure 4].
Conclusion: Our data highlights the importance of serial dd-cfDNA monitoring in allowing for earlier identification of evolving injury and reliable assessment of rejection treatment response following SPKTx.
[1] Simultaneous Pancreas and Kidney Transplant
[2] Biomarkers
[3] Graft Rejection
