Tate Erickson, Canada has been granted the TTS-CST Basic and Translational Mentee-Mentor Award
ABO-incompatible transplantation following enzymatic A-antigen removal in a mouse model: A-antigen re-expression and prevention of early antibody-mediated rejection
Tate Erickson1,2,3, Bruce Motyka1,2,3, Kesheng Tao1,2,3, Jean Pearcey1,2,3, Peter Rahfeld4, Jayachandran N Kizhakkedathu5, Marcelo Cypel2,6, Peter J Cowan7, Stephen G Withers8, Lori J West1,2,3.
1Pediatrics, University of Alberta, Edmonton, AB, Canada; 2Canadian Donation and Transplantation Research Program, Edmonton, AB, Canada; 3Alberta Transplant Institute, Edmonton, AB, Canada; 4Avivo Biomedical Inc, Vancouver, BC, Canada; 5Pathology and Laboratory Medicine, University of British Columbia, Vancouver, BC, Canada; 6Surgery, University of Toronto, Toronto, ON, Canada; 7St Vincent’s Hospital Melbourne, Melbourne, Australia; 8Chemistry, University of British Columbia, Vancouver, BC, Canada
Introduction: The ABO histo-blood group barrier challenges equitable organ allocation due to high risk of rapid antibody-mediated rejection (AMR) of ABO-incompatible transplants (ABOi Tx). Enzymatic reduction of graft ABO-A-antigen (Ag) using FpGalNAc deacetylase and FpGalactosaminidase, termed Azymes, has demonstrated efficacy in converting human ABO-A lungs to ABO-O via ex vivo perfusion. The timing of A-Ag re-expression in the donor organ and the effectiveness of Azymes treatment in preventing early AMR remain unclear. A-transgenic (A-Tg) mice constitutively express A-Ag on vascular endothelium and can be used to model ABOi Tx and AMR. Herein, we used this model to evaluate A-Ag re-expression kinetics after Azyme treatment and determine whether early AMR is prevented by pre-transplant donor organ Azyme treatment.
Methods: After i.v. administration of 0.8mg/kg Azyme (or control), heart and lung from A-Tg C57BL/6 (B6) mice (male/female, n=5/6; 15-19 weeks of age) were harvested. To assess A-Ag removal, organs were stored in organ preservation (UW) solution at 4°C for 4 or 24 hrs then placed in fixative for microscopy. To analyze A-Ag re-expression, hearts were stored in UW solution at 4°C for 4 hrs then heterotopically Tx’d into sex-matched wild-type (WT) B6 recipients and harvested 1, 4 or 7 days later. To model AMR, WT recipients were Tx’d with A-Tg heart (+/- Azyme treatment) followed by i.v. administration of mouse IgM anti-A monoclonal antibody and rabbit complement. Graft function was assessed by palpation 24 hrs post-Tx; A- and H-Ag expression and C4d deposition were assessed by immunohistochemistry.
Results: After in vivo Azyme/4 hrs cold storage, vascular endothelium of A-Tg heart and lung showed marked reduction in A-Ag and were H-Ag positive (Fig 1). Further reduction in A-Ag was observed after 24 hrs cold storage. 24 hours post-Tx, Azyme-treated A-Tg grafts remained H-antigen positive with only very weak/diffuse A-Ag staining (Fig 2A). Azyme-treated hearts at 4 days post-Tx showed weak A-Ag staining primarily localized to outer myocardial microvasculature; at 7 days post-Tx, A-Ag expression was similar to non-Azyme-treated grafts. In the AMR model, the Azyme-treated graft showed no evidence of AMR after 24 hrs, whereas grafts without treatment had reduced graft function, C4d deposition (Fig 2B), and lymphocyte infiltration.
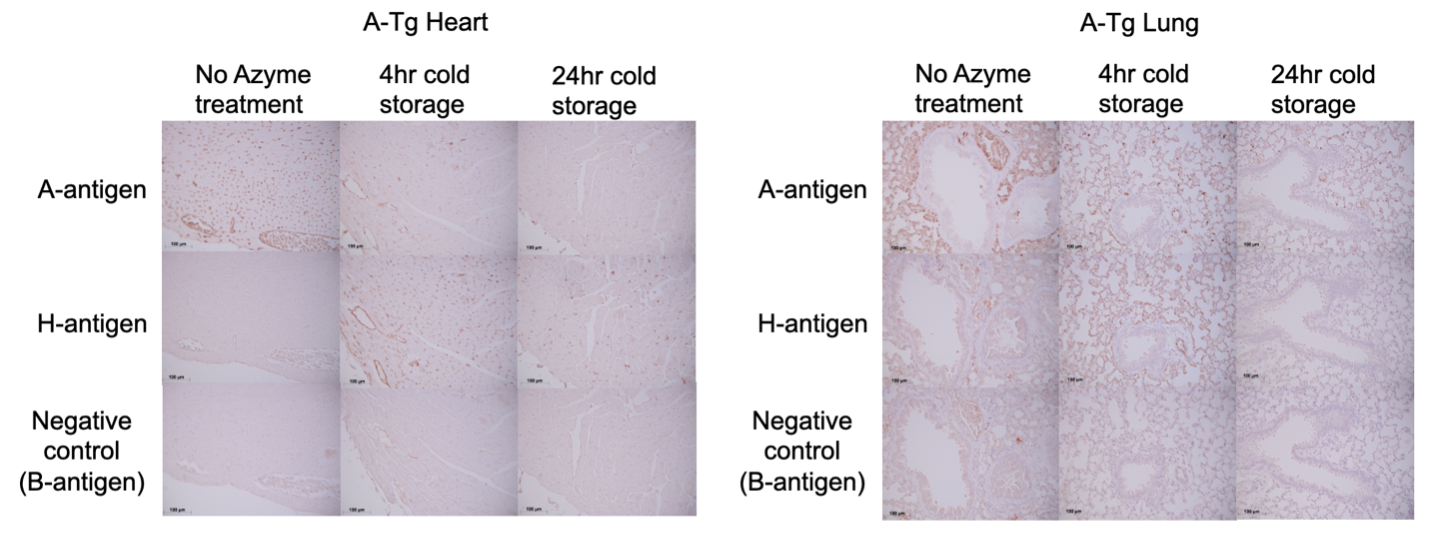
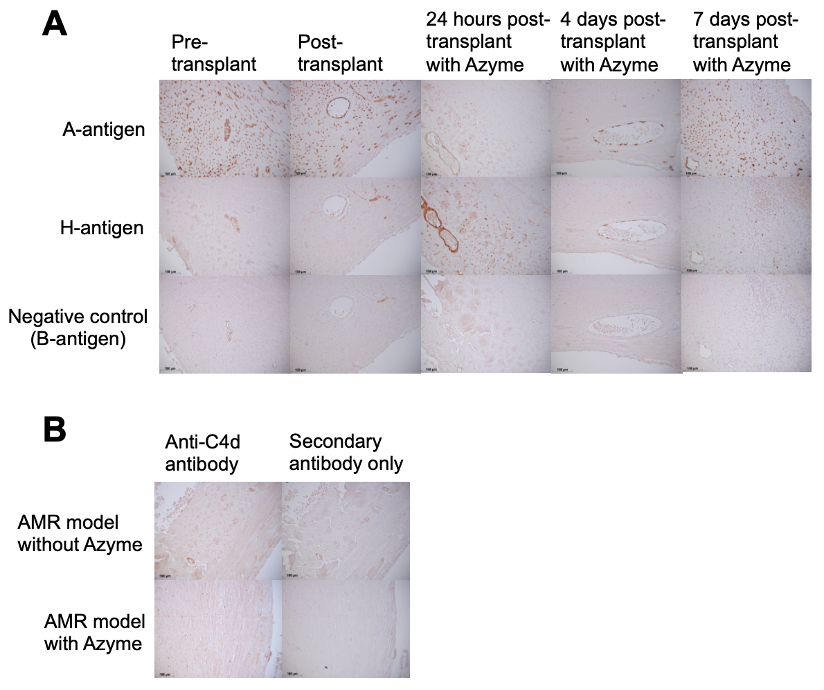
Conclusion: Azyme treatment rapidly depleted A-Ag in A-Tg mouse heart and lung. A-Ag re-expression was minimal in heart grafts 4 days post-Tx, but fully visible 7 days post-Tx. Preliminary findings showed that A-Ag removal by Azyme treatment prevented early AMR. Longer term AMR studies will offer further insight into the effectiveness of temporary A-Ag depletion in avoiding AMR. Clinical application of Azymes may permit additional ABOi Tx, offering lifesaving treatment for individuals for whom compatible organs may not be found and utilizing organs otherwise discarded.
This work was funded by the Collaborative Health Research Project funds from the Canadian Institutes of Health Research (CIHR) and the Natural Sciences and Engineering Research Council of Canada (NSERC). TE was supported through a NSERC Undergraduate Student Research Award.
[1] ABO histo-blood group
[2] enzymatic cleavage
[3] mouse model
