A novel method for hepatocyte transplantation at the liver surface
Takumi Katano1, Akiko Inagaki1, Takehiro Imura1, Hiroki Yamana2, Ryusuke Saito2, Yukiko Endo Kumata2, Shoki Suzuki2, Yoshiya Hagiwara2, Kazuo Ohashi3, Kimiko Watanabe1, Yasuhiko Tabata4, Masafumi Goto1,2.
1Division of Transplantation and Regenerative Medicine, Tohoku University Graduate School of Medicine, Sendai, Japan; 2Department of Surgery, Tohoku University Graduate School of Medicine, Sendai, Japan; 3Graduate School and School of Pharmaceutical Sciences, Osaka University, Osaka, Japan; 4Laboratory of Biomaterials, Department of Regeneration Science and Engineering, Institute for Life and Medical Sciences (LiMe), Kyoto University, Kyoto, Japan
Introduction: Hepatocyte transplantation (HTx) is a promising alternative to liver transplantation; however, poor engraftment is a major challenge. Although co-transplantation with adipose tissue-derived stem cells (ADSCs) or pancreatic islets has been shown to improve engraftment, exposure of these cells to the portal vein flow enhances the instant blood-mediated inflammatory reaction (IBMIR), resulting in a significant loss of transplanted hepatocytes. Therefore, we herein investigated the feasibility of HTx at the liver surface as a novel method that does not involve the portal vein.
Method: Hepatocytes were transplanted onto the liver surface of syngeneic analbuminemic rats with or without ADSCs and/or islets. Serum albumin levels and immunohistochemical staining of transplanted hepatocytes were evaluated. A gelatin hydrogel nonwoven fabric (GHNF) was used as a scaffold for hepatocytes to be transplanted onto the liver surface. Hepatocyte engraftment was also compared between the liver surface and intraportal groups. To investigate the detailed mechanisms behind co-transplantation, co-cultured supernatants were analyzed using a multiplex assay, and an inhibition test using neutralizing antibodies for target factors was performed.
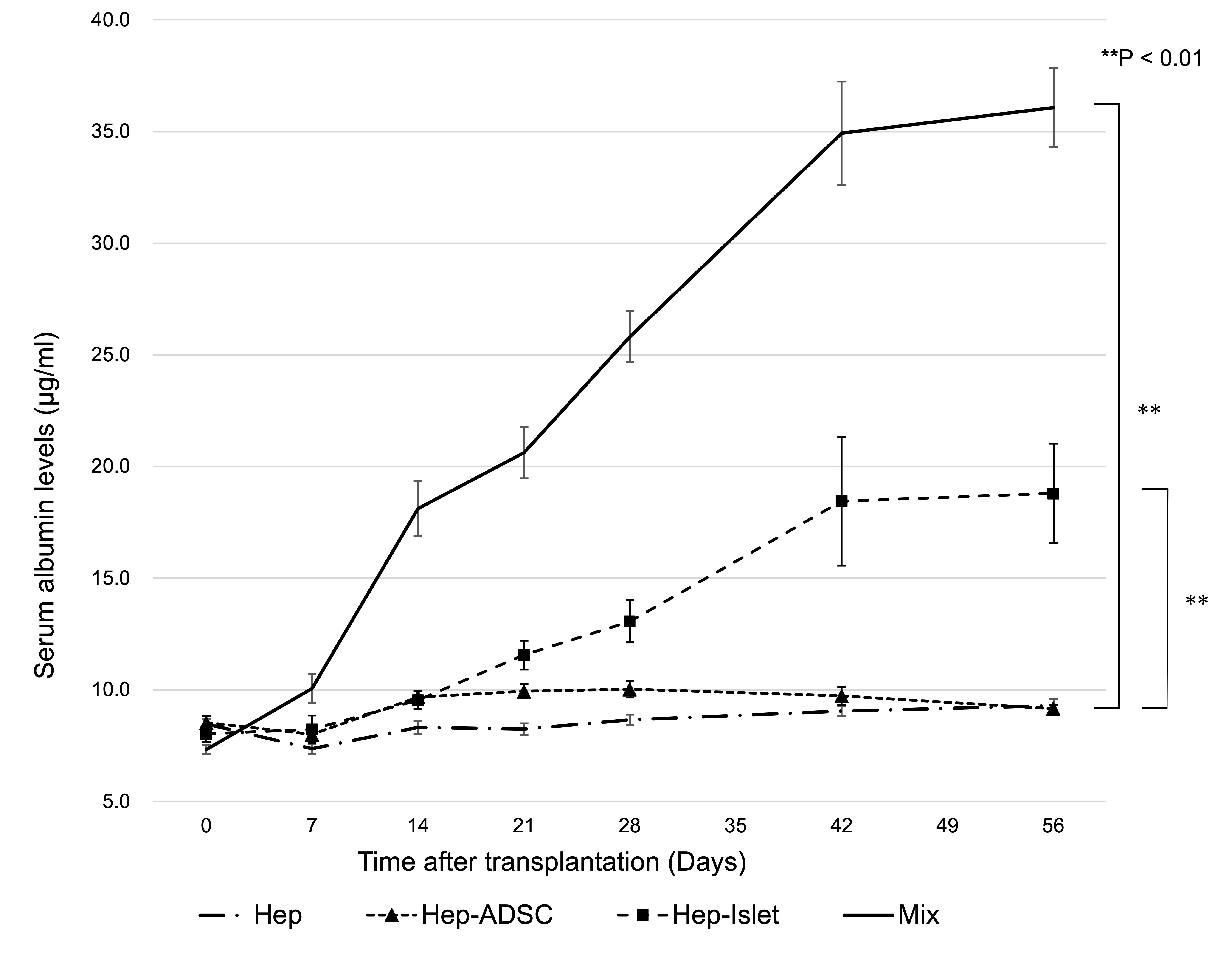
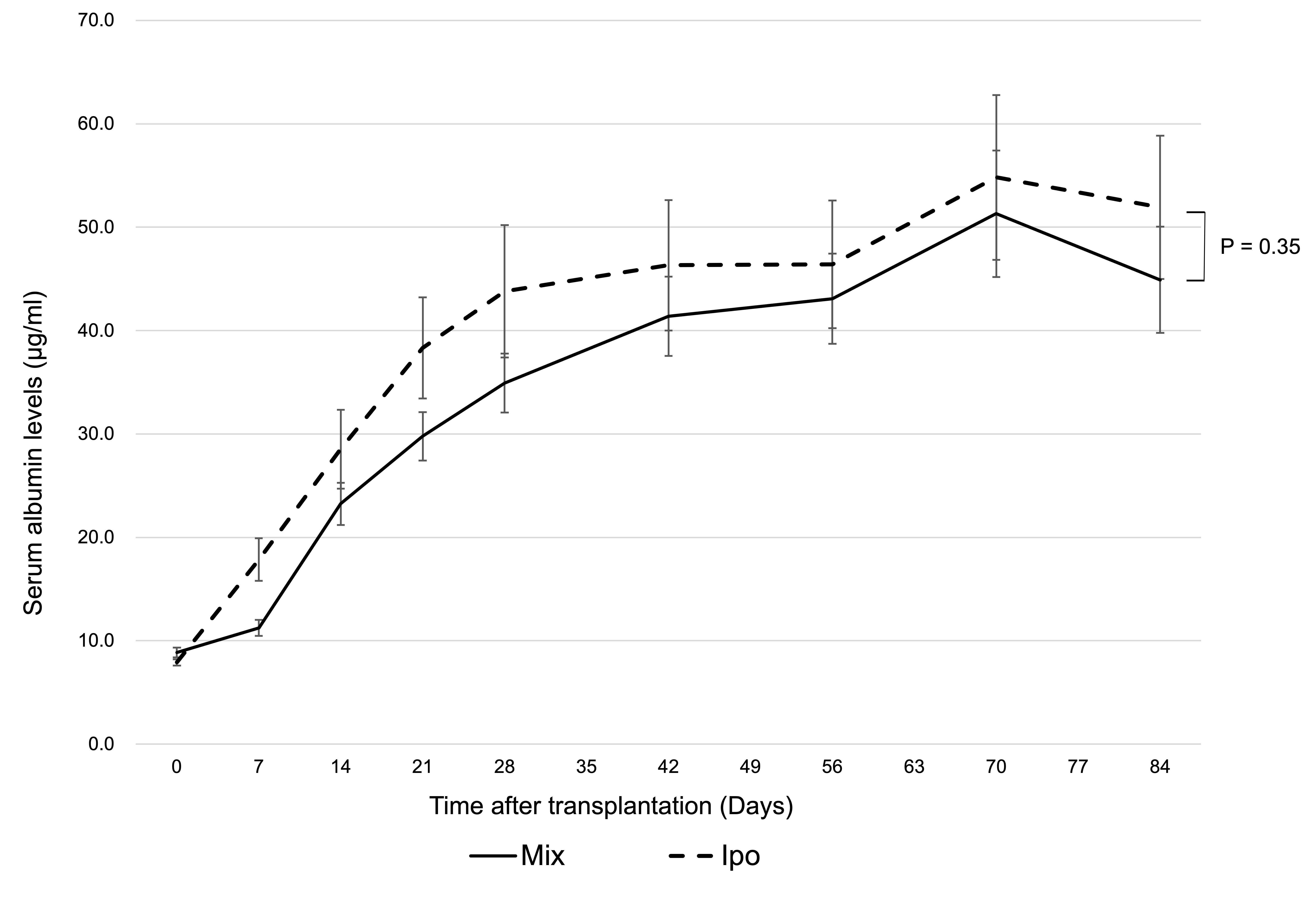
Results: Islet and ADSC co-transplantation markedly enhanced hepatocyte engraftment at the liver surface (P<0.01) (Figure 1; Hep: transplantation of hepatocytes alone, Hep-ADSC: hepatocyte co-transplantation with ADSCs, Hep-Islet: hepatocyte co-transplantation with islets, Mix: hepatocyte co-transplantation with ADSCs and islets), and its efficiency was comparable to that of intraportal transplantation with hepatocyte alone (P=0.35) (Figure 2; Mix: hepatocyte co-transplantation with ADSCs and islets using two GHNF sheets, Ipo: intraportal hepatocyte transplantation). In the co-transplantation group, each cell was not necessarily in close proximity, suggesting that humoral factors rather than cell-cell contact are important. In a co-culture in vitro study, the hepatocyte function was significantly improved by islet and ADSC co-culture (P<0.01). A multiplex assay indicated that VEGF, HGF, LIX, and insulin played a substantial role in enhancing hepatocyte engraftment. Among these factors, insulin appeared to have the most prominent influence according to an inhibition test. Of particular note, inflammatory cytokines were significantly suppressed in the islet and ADSC co-culture group, suggesting that the anti-inflammatory effects of co-transplantation may also contribute to hepatocyte protection.
Conclusion: The present study showed that islet and ADSC co-transplantation using GHNF improved hepatocyte engraftment at the liver surface. HTx at the liver surface together with crucial factors may be a novel alternative strategy for intraportal transplantation to avoid IBMIR.
This study was performed according to a patent application agreement with KYOTO MEDICAL PLANNING Co., Ltd.
[1] hepatocyte transplantation
[2] adipose tissue-derived stem cell
[3] IBMIR
[4] gelatin hydrogel nonwoven fabric
