Exploring metabolic changes in living kidney donors before and after donation: A comparative analysis
Patrícia C Braga1,2, Manuela Almeida1,2,3, Barbara G Carvalho1,2,4, Pedro R Pereira1,2,3, Catarina Ribeiro1,2,3, José Silvano1,2,3, Jorge Malheiro1,2,3, La Salete Martins1,2,3, Marco G Alves5, Anabela S Rodrigues1,2,3.
1Unit for Multidisciplinary Research in Biomedicine (UMIB), Institute of Biomedical Sciences Abel Salazar (ICBAS), University of Porto, Porto, Portugal; 2ITR- Laboratory for Integrative and Translational Research in Population Health, Institute of Biomedical Sciences Abel Salazar , Porto, Portugal; 3Nephrology, ULSde Santo António , Porto, Portugal; 4LAQV-REQUIMTE, Department of Chemistry, University of Aveiro, Aveiro, Portugal; 5Institute of Biomedicine - iBiMED and Department of Medical Sciences, University of Aveiro, Aveiro, Portugal
Living donor (LD) kidney transplant represents the best treatment for end-stage renal disease, but the LD safety is paramount. After kidney donation, glomerular hyperfiltration ensues to compensate for kidney mass loss. Thus, we intend, by metabolic approach, to identify in donor urine samples whether specific patterns are identified and are associated with clinical risk in the donor population. Our study population included 25 patients in pre-donation (T0) and 8 patients after 6 months of donation (T6M).
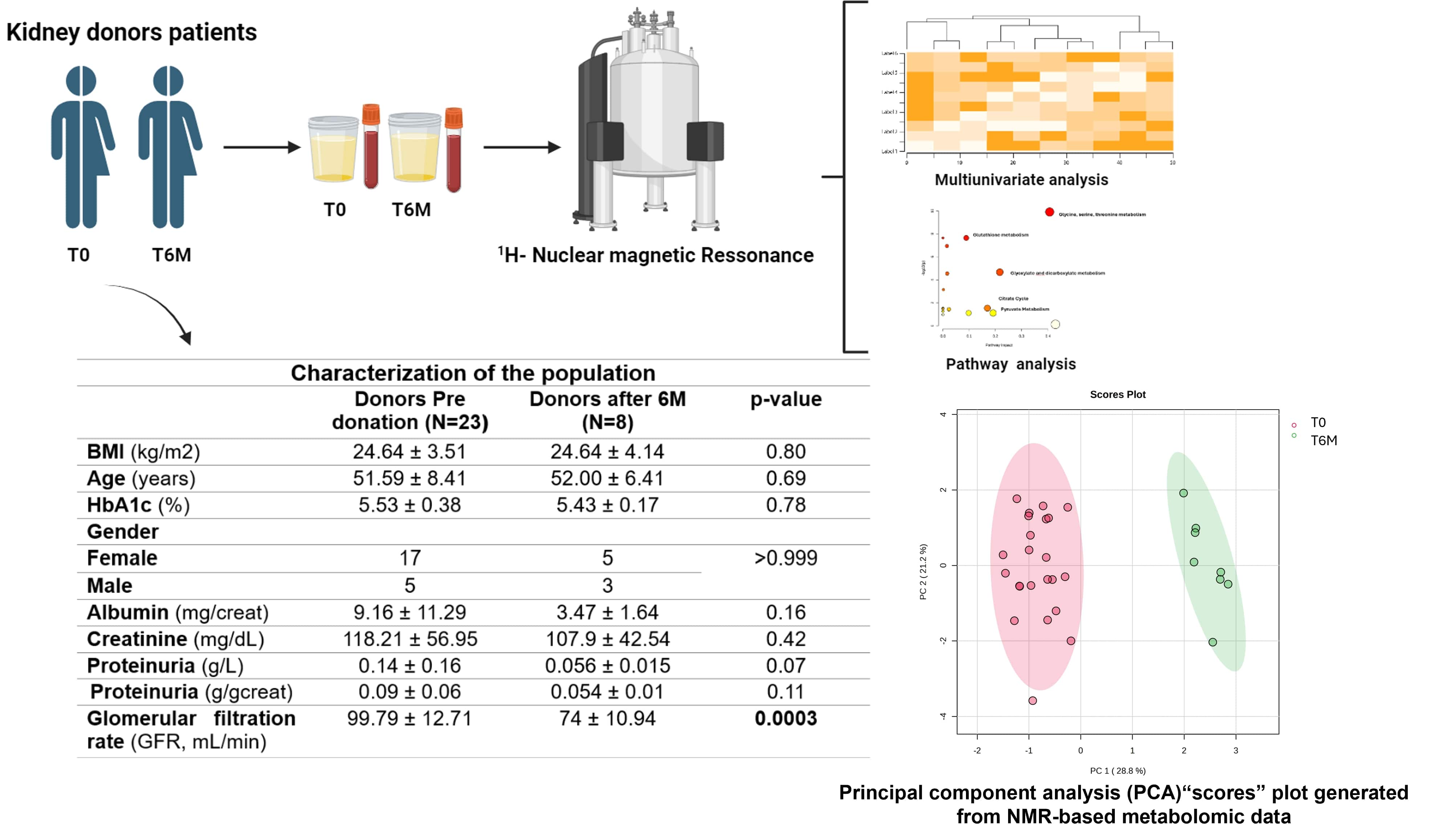
With this approach, we identified 22 metabolites in occasional urine. A principal component analysis (PCA) generated from NMR-based metabolomic data demonstrated that pre and post had significantly different patterns, suggesting the activation of distinct pathophysiological pathways in the early stage after kidney mass loss. Further multivariate analysis revealed that patients (T6M) presented lower values of formate (p=0.048), citrate (p=0.012), dimethylamine (p=0.0003), hypoxanthine (p= 0.001) and betaine (p<0.0001) and higher levels of pyruvate (p<0.0001), glycolate (p=0.0001), sarcosine (p<0.0001) and glycine (p<0.0001), when compared to pre-donation patients.
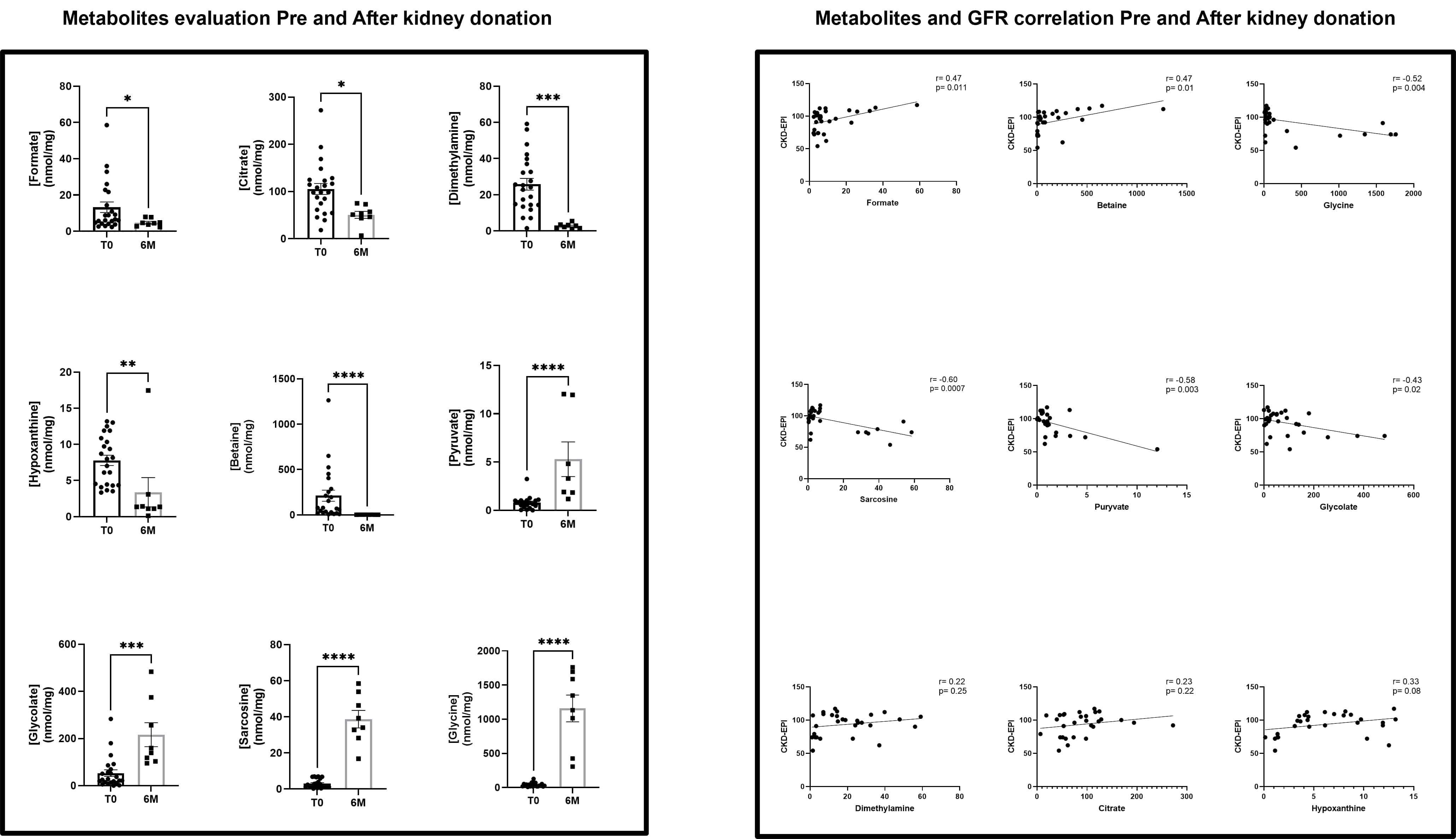
The pathway enrichment analysis results for the dysregulated urinary metabolites in LD revealed the association of the metabolism of mitochondria-associated metabolites, suggesting an impairment of mitochondrial energy production after kidney donation, with the alteration of amino acids that participate on citric acid cycle but also a dysregulation on glycine, glyoxylate and dicarboxylate metabolism Additionally, the patients presented a lower Glomerular filtration Rate (GFR) (74 ± 10.94 vs. 99.79 ± 12.71 mL/min, p=0.0003), and no significant alterations on albuminuria. Also, we observed a strong negative correlation with glycine (r=-0.52, p=0.004), sarcosine (r=-0.60, p=0.0007) and pyruvate (r=-0.58, p= 0.003), a moderate negative correlation with glycolate (r=-0.43, p=0.02), while a moderate positive correlation between GFR and formate and betaine (r=0.47, p=0.01). To further explore biomarkers of renal lesion, we compared the urinary metabolome evaluated of matched samples from individual patients (N=7) pre and post kidney donation. Apart from formate values, which was unaltered in this analysis, the remain metabolites showed results similar to those of the previous investigation. Also, sarcosine and glycolate appeared to have a weak negative correlation with albuminuria (r=0.35 and r=-0.30, respectively). This pilot study suggests that noninvasive evaluation of metabolome in kidney donors, after a certain period of donation, allied with correlation of GFR could be useful to performed clinical risk stratification and tailored management reinforcing kidney donation safety and health gains.
[1] living donors
[2] metabolomics
[3] pilot study
[4] urinary metabolome
[5] mitochondria associated metabolites
