
The role of interleukin 27 in donor specific alloantibody responses
Jose I Valenzuela1, Ran Fan1, Victoria Gorbacheva1, Eduardo Chuluyan2, Robert Fairchild1, Anna Valujskikh1.
1Inflammation and Immunity, Lerner Research Institute, Cleveland Clinic, Cleveland, cleveland, OH, United States; 2CEFYBO, Universidad de Buenos Aires, Buenos Aires, Argentina
Introduction: Antibody-mediated rejection (AMR) is a serious problem in clinical transplantation. There is a great clinical need for the development of efficacious yet safe approaches for inhibiting generation of pathogenic donor specific alloantibodies (DSA) and recipient desensitization. Targeting interleukin-6 (IL-6) showed modest promise for both the AMR treatment and recipient desensitization. However, another member of IL-6 cytokine family, interleukin-27 (IL-27), utilizes the same receptor signaling chain, gp130, and has many overlapping functions with IL-6. Activated dendritic cells (DC) and macrophages were initially identified as major IL-27 producers. However, there is accumulating evidence that other cell types, in particular, B cells, can be an important source of this cytokine. The goal of the current study is to test the role of IL-27 in humoral immune responses using mouse transplant models.
Methods: Heterotopic heart transplant were performed from fully MHC-mismatched BALB/c (H-2d) into B6.WT or B6.IL-27R-/- recipients (H-2b). Blood samples were collected on d. 7, 14, and 21 posttransplant. To test how IL-27 neutralization affects DSA responses full thickness skin from BALB/c mice were grafted onto B6.WT recipients treated with either anti-IL-27 (p28), anti-IL-6, anti-IL-6R or control rat IgG, on day -1, day 3, 7, 11 and 14 after transplantation. Blood samples were collected on d. 7, 14, and 21 posttransplant, and DSA was detected by ELISA for MHC Class I (H-2d) and MHC Class II (I-Ad) antigens. In selected experiments, we used B6.IL27p28Tm reporter recipients in which IL27p28 producing cells can be detected by flow cytometry.
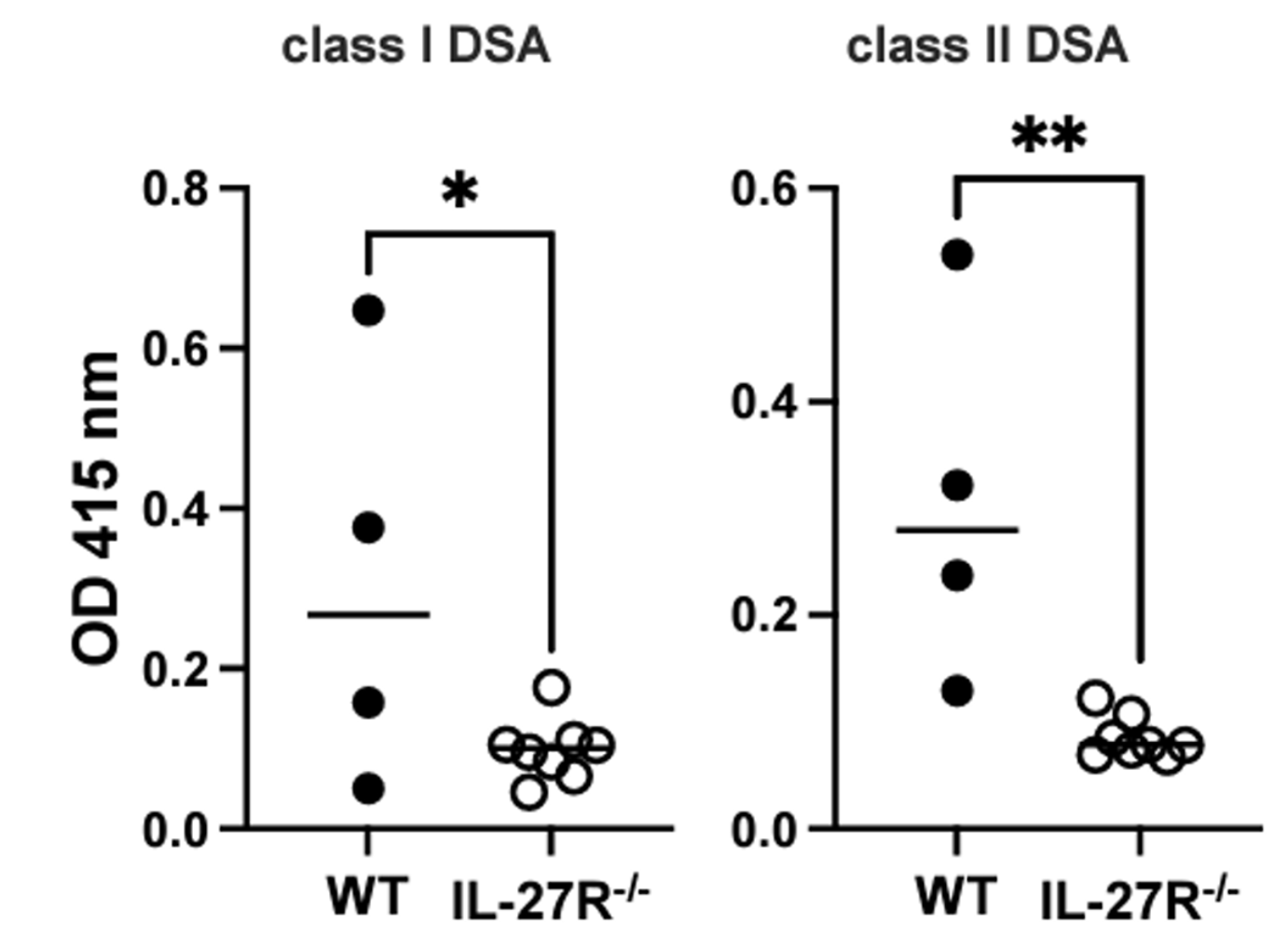
Results: We found that following transplantation, recipient DCs and macrophages produce IL-27. In addition, we detected IL-27 production in various B cell subsets including follicular, marginal zone and transitional B cells, germinal center B cells (B220+CD38-GL7+), and plasma cells at days 7 and 14 following heart transplantation. By d. 21 posttransplant, B6.WT recipients developed serum DSA reactive to donor class I and class II MHC molecules. In contrast, DSA responses were markedly reduced in B6.IL-27R-/- recipients.
While control skin allograft recipients developed anti-H-Dd DSA, none of anti-p28 mAb treated recipients generated DSA, comparable to the effects of anti-IL-6 or anti-IL-6R therapies. Furthermore, anti-IL-27 mAb treatment resulted in a more profound decrease in the numbers of antibody secreting spleen plasma cells (B220+CD138+) compared to anti-IL6 and anti-IL-6R treatments.
Conclusions: Our data suggest that B cells can be an important source of IL-27 following heart transplantation, and identify IL-27 as a therapeutic target for suppressing detrimental DSA responses in transplant recipients.
[1] interleukin
[2] alloantibody
[3] heart transplant
