Kieran Manion, Canada has been granted the TTS-CST Basic and Translational Mentee-Mentor Award
Graft protein shows a distinct signature in donor-specific antibody positive kidney transplant recipients with antibody-mediated rejection
Kieran Manion1, Maya Allen1, Rohan John1, Ana Konvalinka1.
1Toronto General Hospital Research Institute, Toronto, ON, Canada
Introduction: While transplantation is the best treatment for end-stage kidney disease, 50% of grafts fail within 10 years, due mainly to antibody-mediated rejection (ABMR) driven by recipient donor-specific antibodies (DSA). Better strategies are needed to mitigate ABMR, as up to 60% of DSA+ transplant patients do not develop rejection and those with chronic ABMR lack effective treatments. We aim to identify factors regulating kidney protein expression in DSA+ patients with and without rejection.
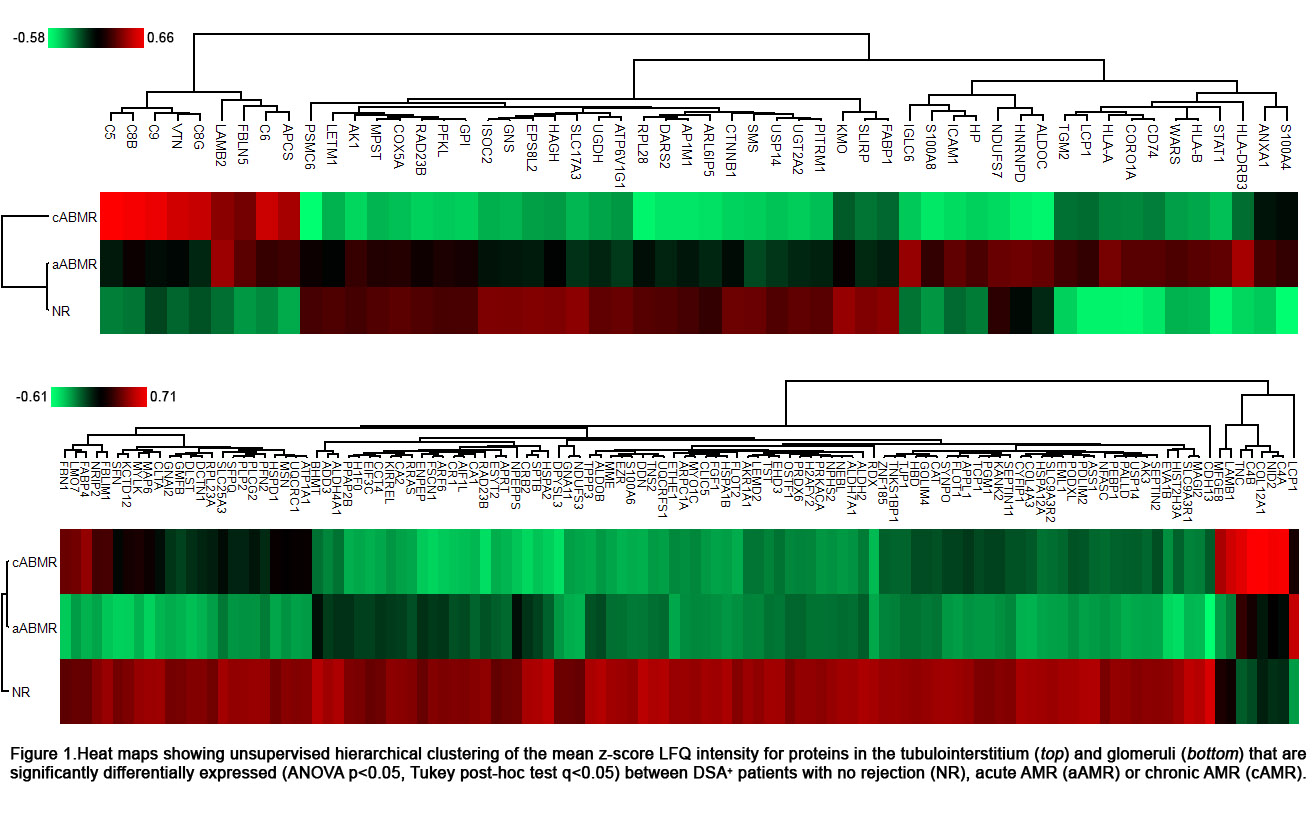
Method: Laser capture microdissection was used to extract glomeruli and tubulointerstitium from formalin-fixed paraffin embedded for-cause kidney biopsies from DSA+ kidney transplant recipients with no rejection (NR, n=45), acute ABMR (aABMR, n=25) and chronic ABMR (cABMR, n=25). Proteins from these tissues were subjected to liquid chromatography mass spectrometry (Q-Exactive HFX) and protein identification was performed using MaxQuant. Perseus software was used to determine significant differential expression within each kidney compartment (ANOVA p<0.05, Tukey post-hoc test q<0.05), and the pathDIP database was used to assess pathway enrichment (FDR q<0.05).
Results: 61 tubulointerstitial and 150 glomerular proteins were significantly differentially expressed between DSA+ patients with NR, aABMR or cABMR (Fig. 1). Patients with cABMR showed significant upregulation of components of the classical complement pathway in the glomeruli (q=6.1x10-5) and of terminal complement in the tubulointerstitium (q=6.3x10-15), as well as significantly decreased expression of tubulointerstitial proteins linked to mitochondrial metabolism (q=1.8x10-5), compared to all other groups. Patients with aABMR showed upregulation of tubulointerstitial proteins involved in MHC (q=3.7x10-6) and IFNγ (8.6x10-6) signaling, as well as decreased expression of glomerular proteins linked to extracellular matrix organization (q=6.7e-3). Lastly, DSA+ NR patients had a significant upregulation of proteins linked to metabolism (TI, q=1.7e-3) and the citric acid cycle (glom, q=2.1e-3), as well as higher expression of podocyte-specific proteins in the glomeruli, as compared to patients with either AMR subtype.
Conclusion: Overall, these results suggest that while acute and chronic ABMR in DSA+ kidney transplant patients both involve concomitant loss of podocyte-specific proteins and metabolic function, the difference between the two ABMR subtypes may stem from the engagement of distinct immune-mediated mechanisms.
