
Stefan G. Tullius, MD, PhD is Professor of Surgery at Harvard Medical School. He is the Joseph E. Murray, MD Distinguished Chair in Transplant Surgery, Chief of Transplant Surgery, and Director of the Transplant Surgery Research Laboratory at Brigham & Women’s Hospital, Boston. He is Vice President of The Transplantation Society (TTS), has served as Sr. Treasurer for TTS, and is a Vice President of the Int’l Society of Uterus Transplantation. He is the incoming Editor-in-Chief of Transplantation and has been is an Executive Editor of Transplantation, an Associate Editor of Transplant International, and has served as Associate Editor of the American Journal of Transplantation. In addition, he leads a productive NIH-funded research laboratory and lists > 300 PubMed scientific manuscripts as well as several book chapters. He is an international speaker at scientific meetings and workshops; serves and has served on the Board of societies such as ESOT, TTS, UNOS, and the National Kidney Registry; and has chaired and co-chaired several committees including the Basic Science Committees for the TTS, AST, and ESOT.
Accelerated aging as a novel concept driving chronic allograft deterioration
Yao Xiao1, Tomohisa Matsunaga1, Keita Nakamori1, Merih Gizlenci1, Friederike Martin1, Elizabeth Zicari1, Reza Abdi2, Yuko Sato1, Hao Zhou1, Stefan Tullius1.
1Division of Transplant Surgery, Transplant Surgery Research Laboratory, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, United States; 2Transplantation Research Center, Renal Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, United States
Purpose: Cellular senescence represents a process in which senescent cells accumulate and secrete an inflammatory senescence-associated secretory phenotype (SASP) that leads to chronic inflammation and fibrosis. We conceptualized chronic allograft deterioration as a process of accelerated aging, driven by chronic inflammation and the activation of fibrogenic signaling. The successful treatment of chronic rejection with the depletion of senescent cells and SASP-related products underscores the relevance of this concept.
Methods and Results: Fully MHC-mismatched heterotopic heart transplantations were performed from BALB/c to C57BL/6 mice. Recipients were treated with CTLA4-Ig to facilitate long-term graft survival while developing characteristic signs of cardiac allograft deterioration. Morphological characteristic signs in parallel to an accumulation of senescent cells and SASP-related inflammation were prominent in chronically rejected cardiac allografts on day 21. Treatment with senolytics (Dasatinib and Quercetin, DQ) reduced cellular infiltrates, depleted senescent cells, and diminished SASP factors. Profiling alloimmune responses revealed systemically increased Tregs with decreased Th1 and Th17 (5.5% vs. 8.1%, 11.1% vs. 5.3%, 9.4% vs. 7.3%, respectively) after DQ treatment; intragraft CD4+ T and CD8+ T cells, neutrophils, and macrophages were reduced (1255 vs. 450, 777 vs. 320, 29 vs. 18, 33 vs. 22 cell counts/mg graft, respectively). Senolytics prolonged graft survival significantly (MST = 34.5 vs. 49.0 days), however, graft fibrosis, a hallmark of chronic rejection, improved only modestly.
Next, we applied a combinatorial treatment with senolytics and the Angiotensin II Receptor blocker Losartan targeting inflammatory and fibrotic signaling. While Losartan alone did not prolong graft survival significantly, the combined treatment extended graft survival in the vast majority to >100 days compared to MST of 34.5 days in controls (Figure 1).
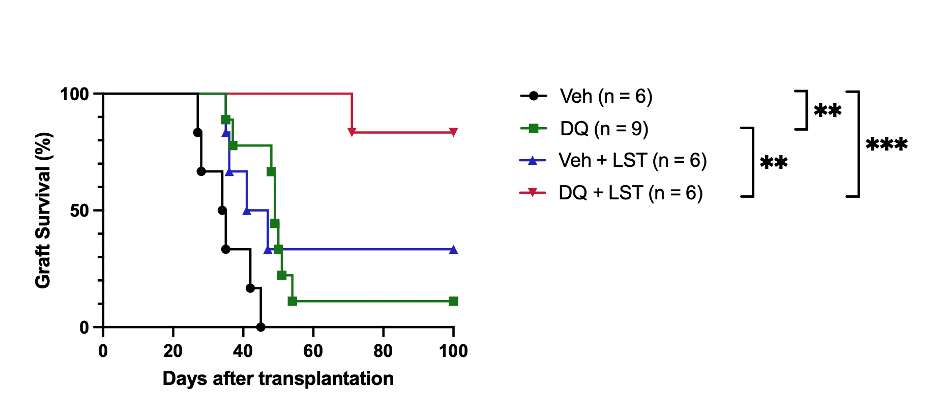
Notably, the combinatorial application of senolytics and Losartan also diminished graft fibrosis, vasculopathy, and cellular infiltration significantly (p < 0.001). Likewise, combinatorial senolytics and Losartan application further reduced intragraft IFNγ+ CD4 T cells while Tregs accumulated (p < 0.001). Mixed lymphocyte reaction (MLR) demonstrated a marked reduction in CD4 and CD8 T cell proliferation (p < 0.001) indicating a dampened alloantigen-specific T cell immune response.
Conclusions: These data document the accelerated aging process as a driver of chronic rejection (Figure 2).
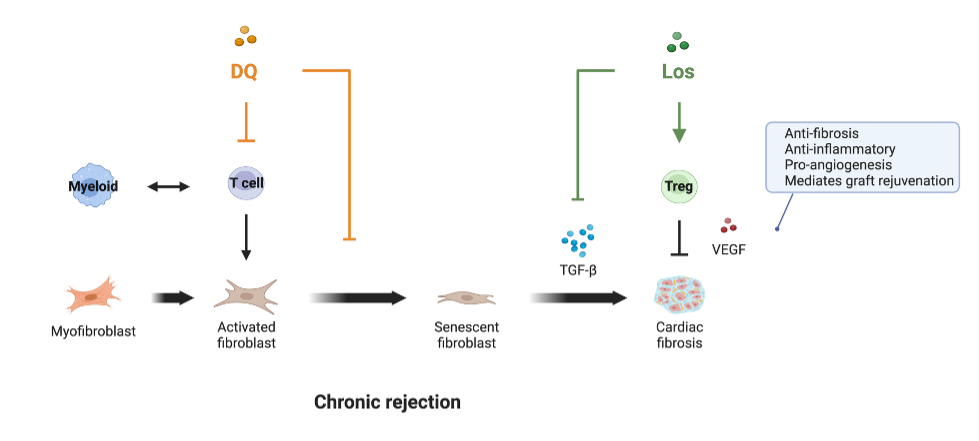
Our study provides compelling evidence that a dual therapy of depleting senescent cells in conjunction with blocking senescence-driven inflammatory/fibrogenic pathways significantly prolongs graft survival while halting fibrosis and graft inflammation.
S.G.T. receives grants from the National Institutes of Health (R01AG064165 and U01AI132898) and is supported by the Pablo and Almudena Legorreta Kidney Health Research Fund. Y.X. and F.M. are supported by the Women in Transplantation Research Fellowship Grant. K.N. and T.M. are supported by the Osaka Medical Foundation Scholarship.
[1] Aging
[2] cellular senescence
[3] chronic rejection
[4] allograft rejection
[5] graft survival
[6] long-term survival
[7] fibrosis
[8] novel therapy
