CD80/CD86-CD28 signal blockade augments the donor-alloantigen-specific inhibitory function of natural regulatory T cells
Kyoko Yogo1, Kazuyoshi Takeda1, Ryuichi Murakami2, Shohei Hori2, Ko Okumura1, Koichiro Uchida1.
1Center for Immunotherapy and Diagnosis, Juntendo University, Tokyo, Japan; 2Laboratory of Immunology and Microbiology, Graduate School of Pharmaceutical Sciences, The University of Tokyo, Tokyo, Japan
Purpose: Our group has reported that co-culture of allogeneic lymphocytes with anti-CD80/CD86 mAbs induced donor-specific immune suppressive function in mice and humans. This approach successfully induced operational tolerance in seven out of ten liver transplant patients. Regulatory T cells (Tregs) have been suggested to be the major population exerting the donor-specific suppressive function. In this study, we investigated the mechanism by which Tregs augmented donor-specific suppressive function during allogeneic co-culture in the presence of anti-CD80/86 mAbs.
Methods: C57BL/6 Foxp3hCD2 mice were used to evaluate suppressive function and the expression of function-associated molecules in Tregs before/after allogeneic co-culture with or without anti-CD80/86 mAbs. Mice expressing a single TCRβ chain were used to analyze the TCRα repertoire of Tregs.
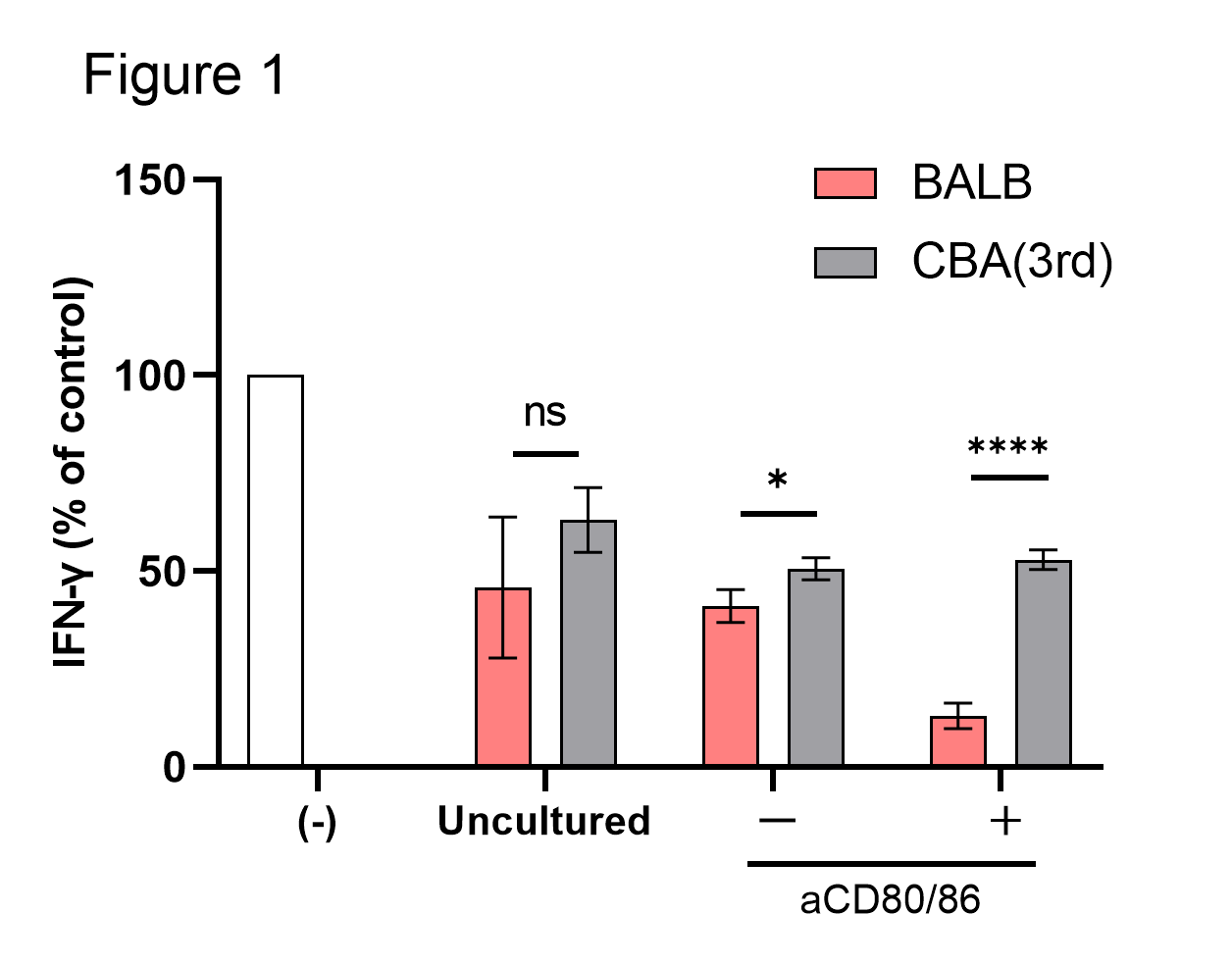
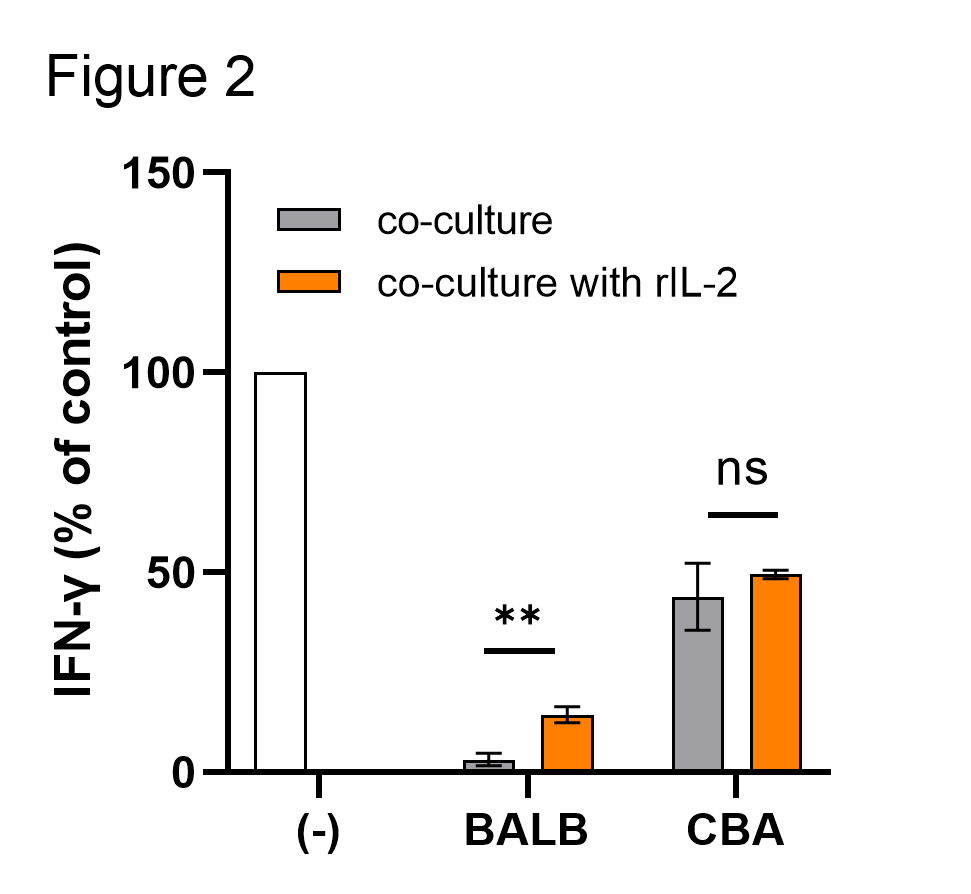
Results: The proliferation of Tregs was remarkably inhibited by anti-CD80/86 mAbs. Anti-CD80/86 mAbs significantly enhanced the suppressive function in a donor-specific manner (Figure 1). An increase in the concentration of IL-2 restored the proliferation of Tregs but inhibited the enhanced donor-specific suppressive function (Figure 2). TCR repertoire analysis showed that several Treg clones were expanded after allogeneic co-culture, but anti-CD80/86 mAbs limited the number of expanded Treg clones. Treg clones expanded during allogeneic co-culture with anti-CD80/86 mAbs were different between donor lymphocytes.
Conclusions: These results suggest that allogeneic co-culture with antagonistic anti-CD80/86 mAbs selectively expanded Tregs expressing high affinity TCRs for donor antigens.
[1] Tolerance
[2] Tregs
[3] Cell therapy
[4] Alloantigen specificity
[5] Costimulatory signal
