Analysis of T-cell alloantigen response via a direct pathway in kidney transplant recipients with donor-specific antibodies
Naoya Iwahara1, Kiyohiko Hotta1, Daiki Iwami2, Tatsu Tanabe1, Takayuki Hirose1, Nobuo Shinohara1.
1Urology, Hokkaido University Hospital, Sapporo, Japan; 2Renal Surgery and Transplantation, Jichi Medical University, Shimotsuke, Japan
Introduction: Donor-specific antibodies (DSAs) are the main cause of graft loss over time. The direct pathway of alloantigen recognition is important in the pathogenesis of acute rejection. Recent studies have suggested that the direct pathway also contributes to the pathogenesis of chronic injury. Nevertheless, there are no reports on T-cell alloantigen response via the direct pathway in kidney recipients with DSAs. In this study, we analyzed the T-cell alloantigen response via the direct pathway in kidney transplant recipients whose graft status was evaluated using graft biopsy.
Method: In total, 96 patients who underwent a human leukocyte antigens incompatible kidney transplantation between 1999 and 2020 were enrolled in the study. Among these recipients, 68 patients were negative for DSAs (DSA−) and 28 patients were positive for DSAs (DSA+). A mixed lymphocyte reaction (MLR) assay was implemented to assess the direct pathway response. To improve the accuracy of T-cell alloreactivity via the direct pathway, we used isolated T cells as responders, and this excluded the T cell response via the indirect pathway.
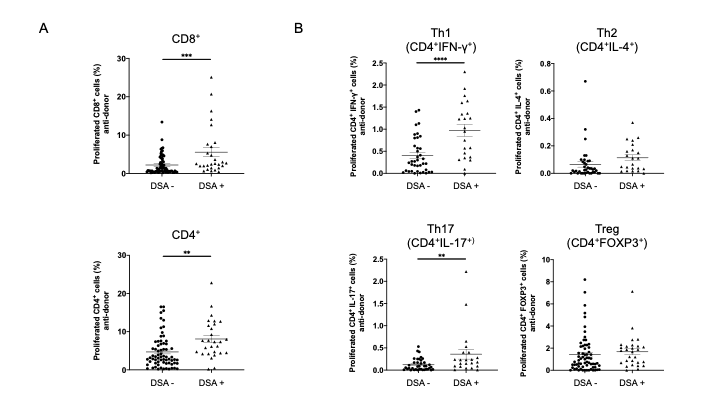
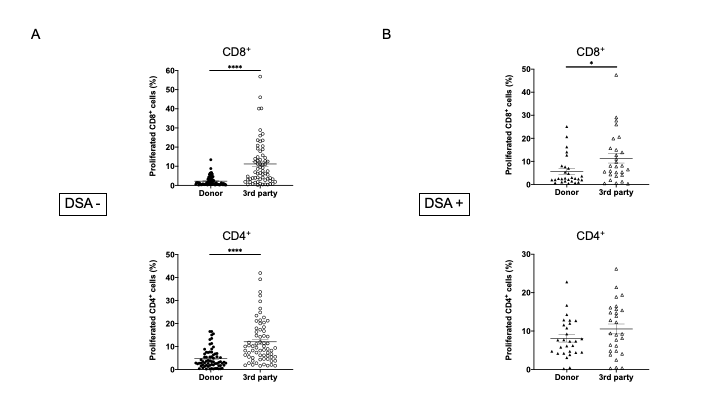
Results: In pathological analyses, none of the patients in the DSA− group showed incidents of rejection, whereas three DSA+ patients developed acute antibody-mediated rejection, 17 DSA+ patients developed chronic antibody-mediated rejection, and 8 DSA+ patients had not yet shown indications of rejection. The MLR assay revealed that the anti-donor CD8+ and CD4+ T cell responses were significantly higher in DSA+ patients than DSA- patients (Figure 1A). To identify CD4+ T cell subsets that showed expansion, responder T cells from DSA− and DSA+ patients were stained for INF-γ, IL-4, IL-17, and FOXP3. Proliferating CD4+ T cells showed a marked increase in Th1 (CD4+INF-γ+) and Th17 (CD4+IL-17+) responses in DSA+ patients than in DSA− patients (Figure 1B). In a comparison between anti-donor and third-party responses, the anti-donor CD8+ and CD4+ T cell response was significantly lower than the anti- third-party response in DSA- patients (Figure 2A). In DSA+ patients, the anti-donor CD8+ T-cell response was significantly lower than the third-party response. In contrast, CD4+ T cell proliferation was similar in the donor and third-party, indicating that donor-specific hyporesponsiveness of CD4+ T cells was absent in DSA + patients (Figure 2B).
Conclusion: Our study demonstrated that DSA+ recipients have a greater potential for developing immune responses against the donor tissues via the direct alloantigen recognition pathway. These data contribute to an understanding of DSAs pathogenicity during kidney transplantation.
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, grant number 18K09156.
[1] donor-specific antibody
[2] direct alloantigen recognition pathway
[3] immune monitoring
[4] mixed lymphocyte reaction
[5] kidney transplantation
