Immunosuppressive drug combinations after kidney transplantation and post-transplant diabetes: A systematic review and meta-analysis
Laia Oliveras1,5, Edoardo Melilli1,5, Adnan Sharif2, Manfred Hecking3, Martina Guthoff4, Josep M Cruzado1,5, Julio Pascual6, Nuria Montero1,5.
1Nephrology, Hospital Universitari de Bellvitge, L'Hospitalet de Llobregat, Spain; 2Nephrology and Transplantation, University Hospitals Birmingham, Birmingham, United Kingdom; 3Internal Medicine III, Clinical Division of Nephrology and Dialysis, Medical University of Vienna, Vienna, Austria; 4Diabetology, Endocrinology, Nephrology, University of Tübingen, Tübingen, Germany; 5Nephrology and Transplantation, Biomedical Research Institute (IDIBELL), L'Hospitalet de Llobregat, Spain; 6Nephrology, Hospital 12 de Octubre, Madrid, Spain
Introduction: Post-transplant diabetes mellitus (PTDM) is a frequent complication after kidney transplantation (KT), with an incidence between 20-30%. PTDM increases the risk of cardiovascular disease, which is one of the main causes of death with functioning grafts. The pathophysiology of PTDM involves beta-cell dysfunction, with impaired insulin secretion and insulin resistance overlaid immunosuppression accelerating preexisting damage. The major risk factor in the post-transplant period is immunosuppression therapy, such as steroids, calcineurin inhibitors (CNI), and mTOR inhibitors (mTORi). It is also a major modifiable risk but must be balanced with rejection risk. This systematic review aims to investigate the effect of different immunosuppressive regimens on the risk of PTDM incidence.
Method: We performed a systematic literature search in MEDLINE and CENTRAL for randomized controlled trials (RCTs) that included KT recipients with any immunosuppression and reported PTDM outcomes up to 1 October 2023. The definition of PTDM was whatever was used by the authors. Meta-analysis was done by pooling data for calculating the relative risks (RR) of the outcome by comparing different immunosuppression strategies.
Results: We identified 5611 reports. After removing duplicates and screening titles and abstracts 325 full-text reports were assessed. We finally included 125 RCTs. We found no differences in PTDM risk within induction therapies. In de novo KT, there was an increased risk of developing PTDM with tacrolimus versus cyclosporin (RR 1.71, 95%CI [1.38-2.11]).
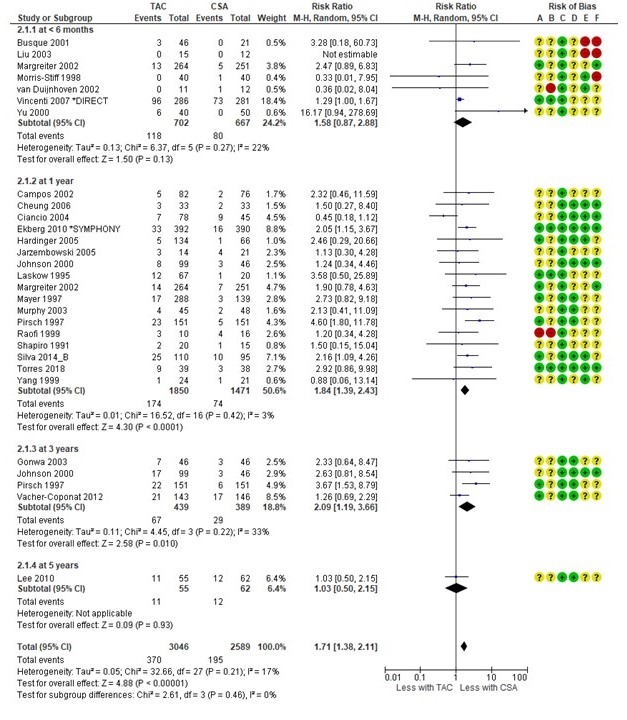
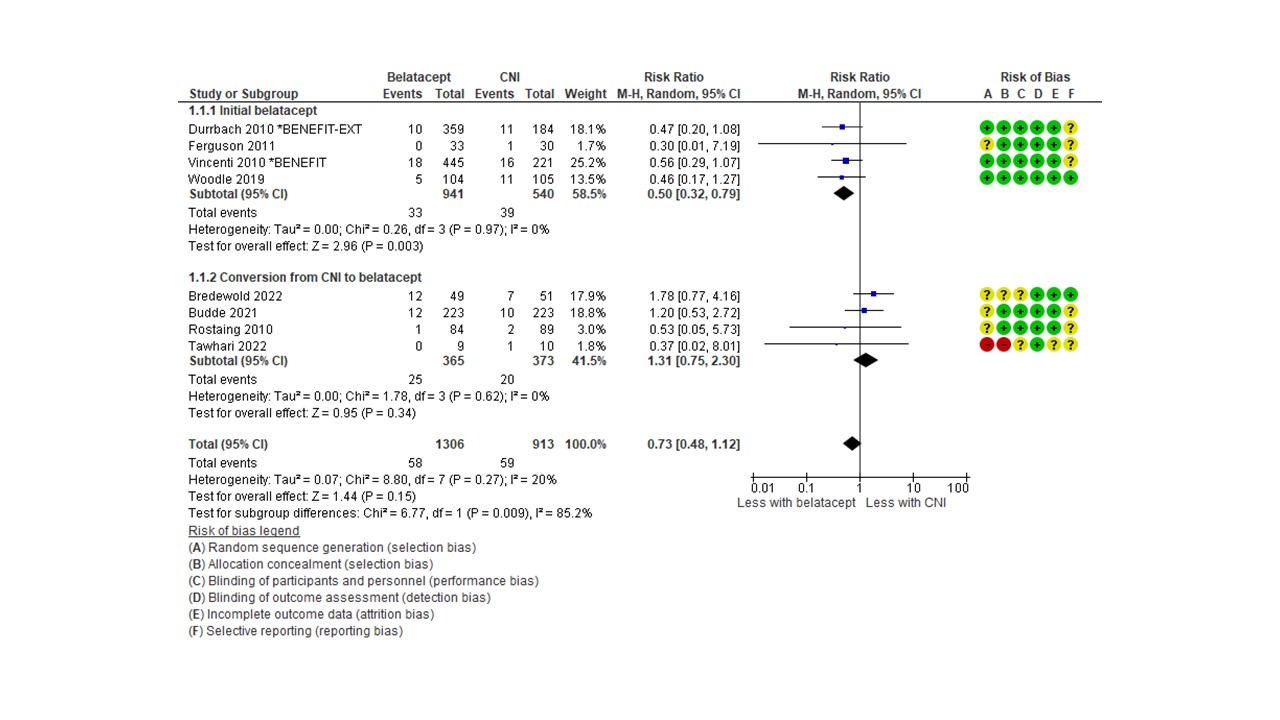
No differences were observed between tacrolimus+mTORi and tacrolimus+MMF/MPA, but there was a tendency towards a higher risk of PTDM in the cyclosporin+mTORi group (RR 1.42, 95%CI [0.99-2.04]). Conversion from cyclosporin to an mTORi increased PTDM risk (RR 1.89, 95%CI [1.18-3.03]). De novo belatacept compared with a calcineurin inhibitor resulted in 50% lower risk of PTDM (RR 0.50, 95%CI [0.32-0.79]).
Steroid avoidance resulted in 31% lower PTDM risk (RR 0.69, 95%CI [0.57-0.83]), whereas steroid withdrawal resulted in no differences.
Conclusion: Immunosuppression should be decided on an individual basis, carefully weighing the risk of future PTDM and rejection.
[1] post-transplant diabetes
[2] kidney transplantation
[3] immunosuppression
[4] metabolic complications
