Donor-derived cell-free DNA identifies subclinical antibody-mediated rejection in de novo donor-specific antibody-positive kidney transplant recipients
Ara Cho1, Ahram Han1, Juhan Lee3, Jae Berm Park4, Cheol Woong Jung5, Yong Chul Kim2, Sehoon Park2, Sangil Min1, Jongwon Ha1.
1Surgery, Seoul National University Hospital, Seoul, Korea; 2Internal Medicine, Seoul National University Hospital, Seoul, Korea; 3Surgery, Severance Hospital, Seoul, Korea; 4Surgery, Samsung Medical Center, Seoul, Korea; 5Surgery, Korea University Medical Center, Seoul, Korea
Introduction: Antibody-mediated rejection (ABMR) remains a significant diagnostic and therapeutic challenge for kidney transplant recipients. While histological examination is the gold standard diagnostic method, it is invasive. Donor-specific antibodies (DSA) and donor-derived cell-free DNA (ddcf-DNA) represent potential alternatives for predicting rejection. The aim of this study is to predict active ABMR through the combination of DSA and ddcf-DNA in kidney transplant recipients who maintain stable renal function after post-transplantation.
Method: A total of 80 kidney transplant recipients aged 18 or older were enrolled across five tertiary hospitals in Korea. We administered serum ddcf-DNA and DSA tests of patients with stable kidney function since February 2023. The histologic results of kidney biopsy were compared with the test outcomes. Patients with ABO or HLA incompatible kidney transplantation were excluded.
Results: We examined a total of 80 patients, with 49 (61.3%) being male, and the mean age was 48 years. Among them, 33 patients (41.3%) showed DSA positivity. In the DSA-positive group, the median ddcfDNA was 1.42% (IQR: 0.65-1.86), while the median ddcfDNA was 0.31% (IQR 0.22, 0.69) in the DSA-negative group. 11 patients were diagnosed of acute ABMR in histology. The area under the curve (AUC) for dnDSA in predicting acute ABMR is 0.741 (95% CI: 0.581-0.902), while for ddcfDNA, it is 0.789 (95% CI: 0.650-0.928). The combination of dnDSA and ddcfDNA yields an AUC of 0.807 (95% CI: 0.659-0.955) for predicting acute ABMR. The positive predictive value of ddcfDNA at 0.7% to detect active ABMR in DSA+ patients was 27.8%, while the negative predictive value was 100.0%.
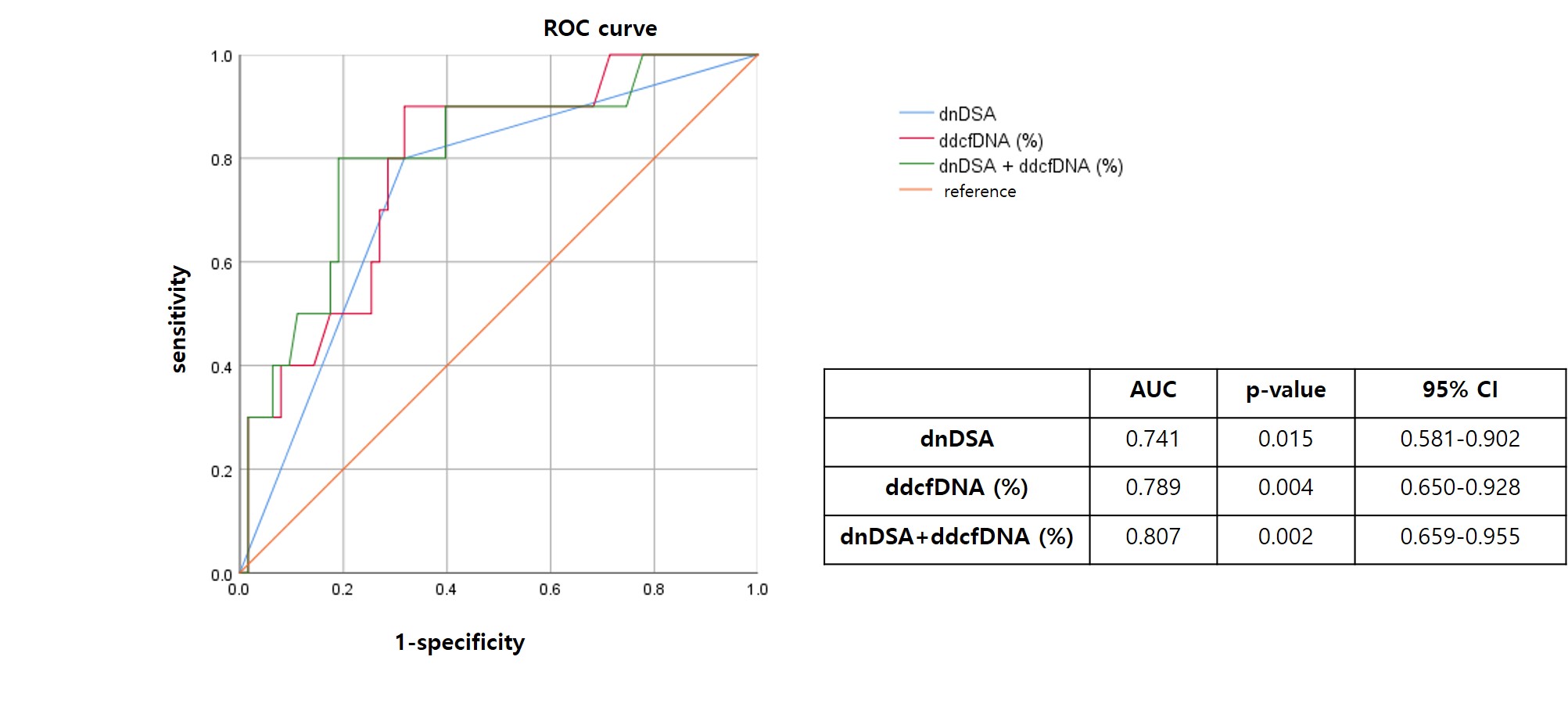
Conclusion: The combination of ddcf-DNA and DSA testing holds value as a noninvasive diagnostic approach for active ABMR in kidney transplant recipients with stable renal function.
This study was funded by BioTIDE Co., Ltd.
[1] Donor derived cell free DNA
[2] Donor specific antibody
[3] Acute antibody mediated rejection
