A prospective observational study to assess the role of donor derived cell free DNA (dd-cfDNA) in predicting active rejection in renal allograft recipients
Debdeep Mondal1,2, Pratik Das1.
1Nephrology , R.N.Tagore International Institute of Cardiac Sciences , Kolkata, India; 2Nephrology , Healthworld Hospitals, Durgapur, Asansol, India
Introduction: Despite significant rise in renal transplantation figures worldwide, the main concern of transplantation is rejection. Allograft biopsy is considered gold standard for diagnosis but often not feasible due to its invasive nature. Rise in serum creatinine is not specific for rejection & often contributed by other factors also. Donor derived cell free DNA (dd-cfDNA) are found in blood and urine originated from allograft cells in the setting of allograft injury serves as nonivasive biomarker. Lack of data in renal allograft recipients (specially in Indian subcontinent) prompted us to do the study.
Method: In this prospective observational single centre study 45 patients were evaluated within 1 year of study period. cf-DNA was isolated from plasma and result was represented as % of dd-cfDNA in total cfDNA sample. Levels of plasma dd-cfDNA & serum creatinine compared with allograft rejection status ascertained by histology in biopsy specimens (32 indication biopsies,13 protocol biopsies) and analysed statistically.
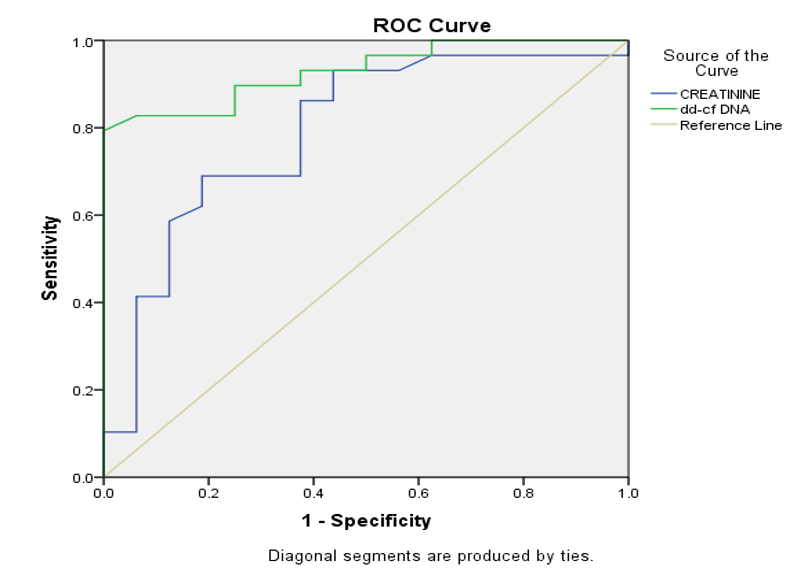
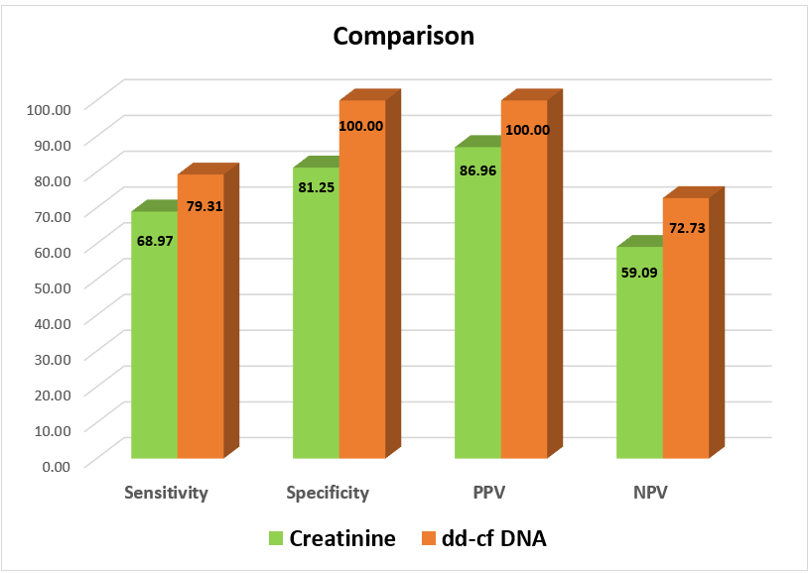
Result: 29 (out of 45) patients(64.4%) had biopsy proven rejection(ABMR-51.8%,TCMR-24.1%,Mixed rejection-24.1%)Other findings: ATN(6.7%),CNI toxicity(4.4%),recurrence of basic disease(2.2%) & IFTA dd-cfDNA levels discriminated rejection with ROC-AUC of 0.9 and provided an estimated NPV 72.73% & PPV 100 % at cut off of 0.83% dd-cfDNA & had 100% specificity & 79.31% sensitivity to discriminate rejection from non-rejection(all parameters better than creatinine).
dd-cfDNA was significantly different in median values among ABMR,TCMR & Mixed rejection
dd-cfDNA levels discriminated ABMR from TCMR with ROC-AUC of 0.95 at a cut off of 1.14% however serum creatinine failed to do so dd-cfDNA detects Active ABMR at a lower cut off value of 0.75% (ROC-AUC of 0.995) (as compared to 0.83% for whole rejection group) probably due to widespread tissue injury dd-cfDNA levels discriminated Chronic active ABMR with ROC-AUC of 1.0 at a cut off of 0.83% from non-rejection group.
No statistically significant difference between serum creatinine and dd-cfDNA in Active ABMR and Chronic active ABMR, hence they can't differentiate between them.
Conclusion: dd-cfDNA can be used as a noninvasive biomarker as it can discriminate between rejection & nonrejection with a cut off of 0.83% with better sensitivity, specificity, PPV & NPV than serum creatinine.
The elevation of ddcfDNA is significantly higher in ABMR and mixed rejection than in TCMR.
Immensely helpful in predicting rejection when there is contraindication for biopsy or unwillingness for invasive procedure.
Useful in detecting rejection when histology are not conclusive for active ABMR but there is strong suspicion for ABMR(e.g unexplained ATN in presence of DSA).
Useful as a screening tool for monitoring rejection in absence of clinically evident graft dysfunction.
In future largescale studies needed to explore its potential as noninvasive biomarker.
[1] donor derived cell free DNA , dd-cfDNA, rejection , renal transplant, antibody mediated rejection , renal allograft biopsy , noninvasive biomarker , creatinine
