
Long-term outcomes of kidney transplant recipients from deceased donors with circulatory determination of death
Lawrence Slapcoff1, Emilie Trinh1, Shaifali Sandal1, Sonali de Chickera1, Prosanto Chaudhury1, Steven Paraskevas1, Jean Tchervenkov1, Marcelo Cantarovich1.
1McGill University Health Centre, Montreal, Canada
Background: As per the Global Observatory on Donation and Transplantation, < 10% of the global needs for kidney transplantation (KTx) are met. Donors with circulatory determination of death (DCD) represented 23% of the transplanted organs in 2022. Increased KTx from these donors has the potential to increase the donor pool; however, concerns remain regarding the long-term outcomes associated with their use.
Aim: To compare clinical outcomes in recipients of KTx from brain death donors (DBD) vs. DCD at a single academic center.
Methods: We performed a retrospective cohort study of all adult KTx recipients from deceased donors, from Sept 1, 2011 to Sept 30, 2022. Patients were classified into four subgroups: DBD-SCD (standard criteria donor), DBD-ECD (expanded criteria donor), DCD non-ECD, and DCD-ECD. The primary outcome was death censored graft survival (DCGS). Secondary outcomes included: Primary non-function (PNF), delayed graft function (DGF), death with graft function, incidence of first acute rejection and estimated glomerular filtration (eGFR) rate over time.
Results: We included 940 patients, 757 (80.4%) with KTx from DBD and 183 (19.6%) from DCD. 65.2% were male. We excluded 170 living donor recipients. Amongst the DBD kidneys, 369 (48.7%) were from SCD and 388 (51.3%) from ECD. Among the DCD kidneys, 114 (62.3%) were from non-ECD, and 69 (37.7%) from ECD. DCD kidneys included 37 (20%) from donors having medical assistance in dying (MAiD). Immunosuppression consisted of alemtuzumab induction and maintenance tacrolimus and mycophenolate sodium. Maintenance prednisone was used in highly sensitized patients. At 10 years, DCGS differed significantly among the four subgroups: 78.8% in DBD-SCD, 70.2% in DBD-ECD, 80.4% in DCD non-ECD, and 69.8% in DCD-ECD (p<0.01). PNF did not differ significantly between groups: 1.63% in DBD-SCD, 4.38% in DBD-ECD, 3.51% in DCD non-ECD, and 5.8% in DCD-ECD (p=0.11). The incidence of DGF was as follows: 22.8% in DBD-SCD, 23.2% in DBD-ECD, 45.6% in DCD non-ECD, and 44.9% in DCD-ECD (p<0.01). Death with graft function at 10-years differed significantly among the four subgroups: 21.5% in DBD-SCD, 48.8% in DBD-ECD, 11.6% in DCD non-ECD, and 34.6% in DCD-ECD (p<0.01). The incidence of acute rejection at 1-year was 8.5% in DBD-SCD, 15.4% in DBD-ECD, 14.2% in DCD non-ECD, and 10.7% in DCD-ECD (p=0.09). Median eGFR (ml/min/1.73 m2) at 1-, 5- and 10-years differed between subgroups: DBD-SCD (60, 55, 43), DCD-ECD (40, 35, 22), DCD non-ECD (53, 59, 53), and DBD-ECD (41, 38, 44) (1-year: p<0.01, 5-years: p<0.01, and 10-years: p=0.23). Among the MAiD donor recipients, DCGS was 79.3% and death with graft function was 8% at 5-years.
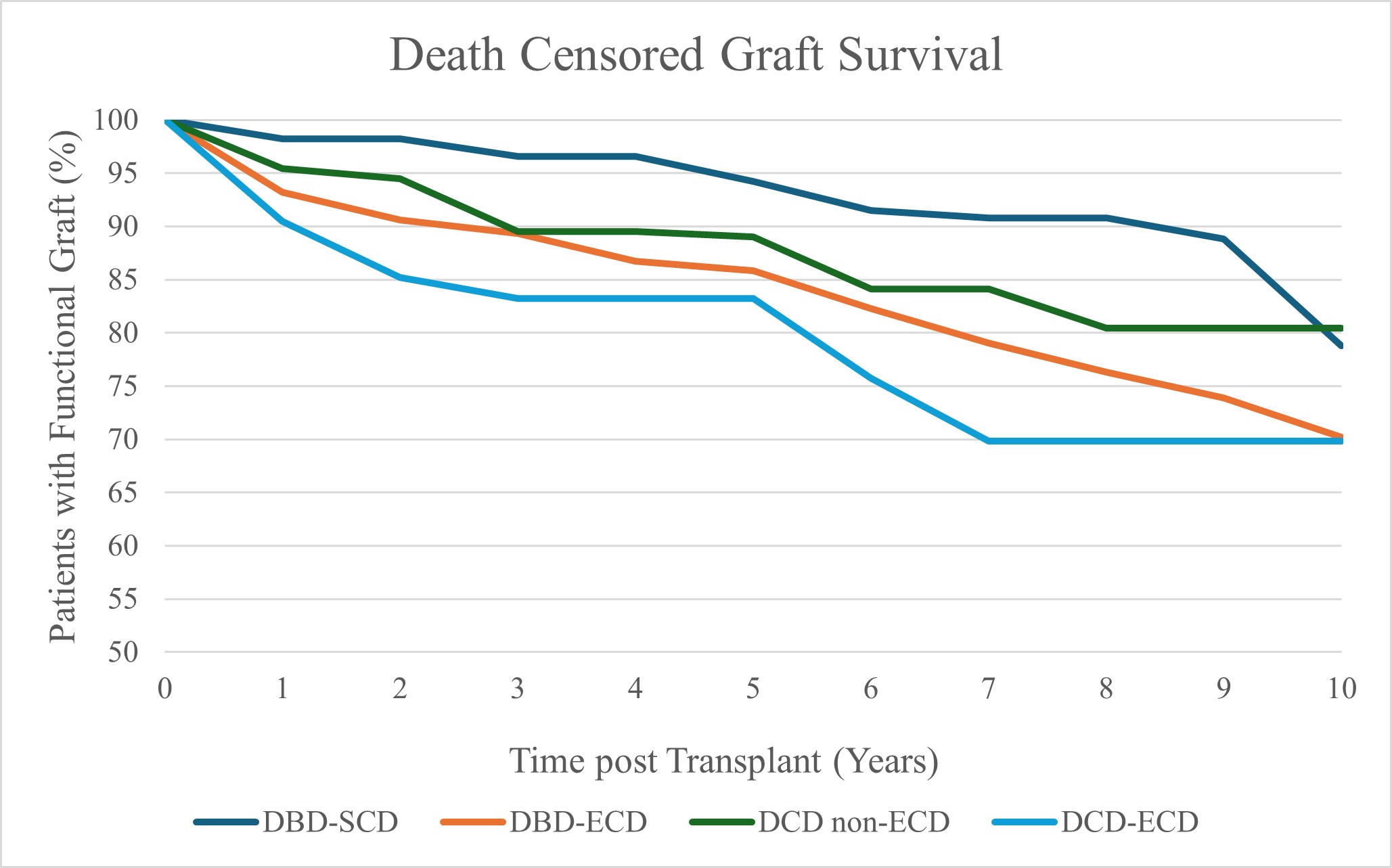
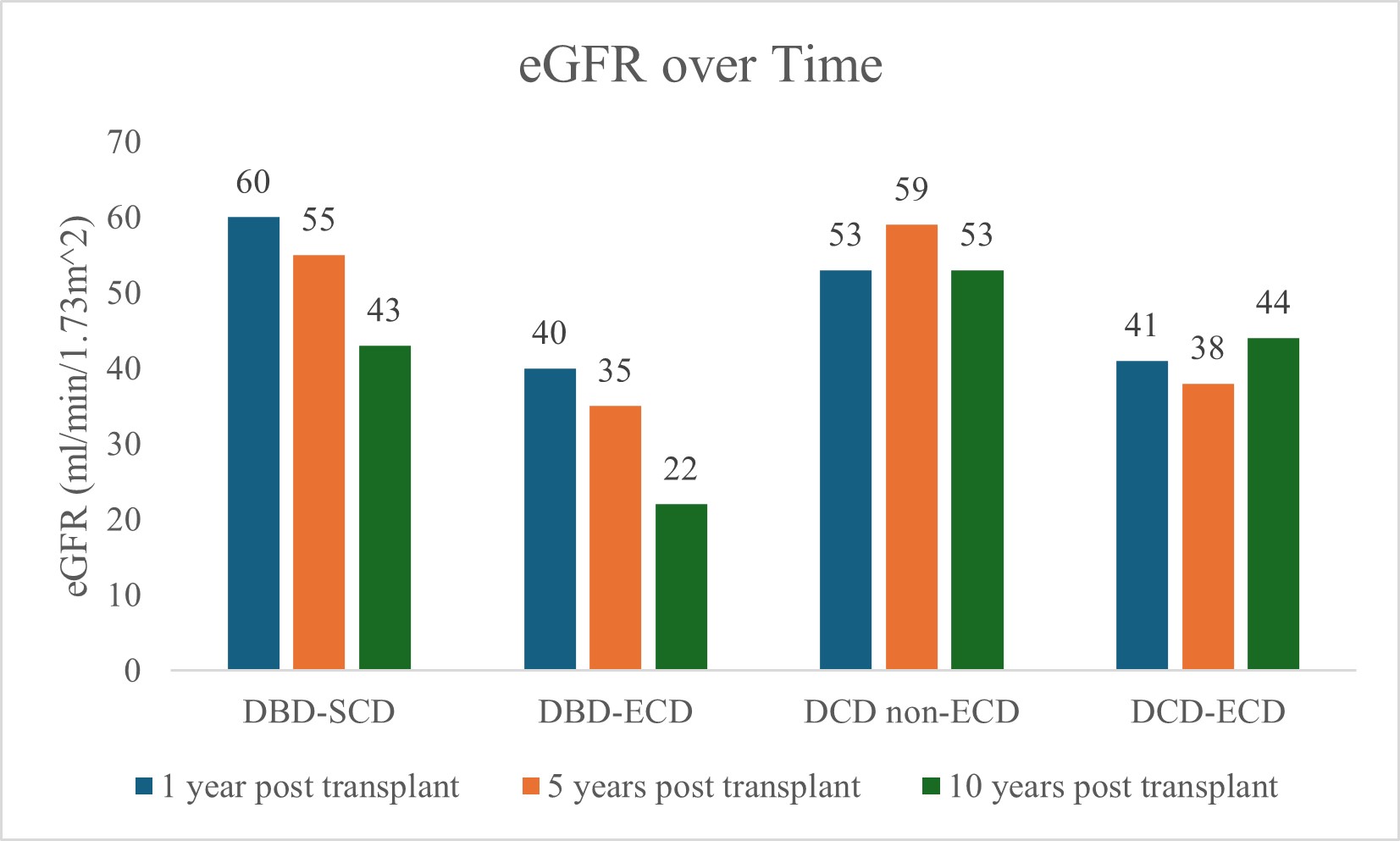
Conclusions: DCD donors remain a critical source of transplantable kidneys to address global needs. Our findings contribute important long-term data to support their use.
[1] Donor Evaluation
[2] Kidney Transplanation
[3] Circulatory Determination of Death
[4] Long Term Outcomes
[5] Graft Survival
