Plasma cell-rich rejection significantly impacts graft survival in paediatric liver transplantation recipients
Ryan Gemell1, Priya P Walabh2, Christina C Hajinicolaou2, Martin M Hale3.
1Department of Paediatrics and Child Health, University of the Witwatersrand, Johannesburg, South Africa; 2Department of Paediatrics and Child Health, Division of Paediatric Gastroenterology, University of the Witwatersrand , Johannesburg, South Africa; 3Department of Anatomical Pathology, School of Pathology , Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
Introduction: Liver transplantation is the standard of care for paediatric patients with cirrhosis, irreversible acute liver failure, metabolic liver disease and malignancy. Plasma-cell rich rejection (PCRR), formerly known as de novo autoimmune or plasma cell hepatitis, is a poorly described, form of late-onset (> 6 months) antibody-mediated liver rejection. The study aimed to analyse the clinicopathological factors associated with PCRR in a paediatric liver transplant unit in Johannesburg, South Africa.
Methods: A retrospective analysis of records of 91 paediatric liver transplant recipients ≤18 years of age who underwent liver transplantation between 1 January 2015 and 31 December 2021 at the Wits Donald Gordon Medical Centre (WDGMC) and followed up at Charlotte Maxeke Johannesburg Academic Hospital (CMJAH) liver transplant clinic was conducted. Ethics approval was obtained from the University of the Witwatersrand Human Research Ethics Committee (HREC), M Ethics number: M2211108. Data collected included demographic characteristics, acute cellular rejection (ACR) episodes, Banff scoring of ACR episodes, histological characteristics and post-transplant complications like biliary strictures.
Results: In the study period, 91 patients underwent paediatric liver transplantation (PLT). In the PLT patients, 45/91 (49, 5 %) developed ACR due to poor adherence to immunosuppression as reflected by a Mean Level Variability Index (MLVI) of 2.39, SD=1.78, (p=0.015). Late-onset acute rejection (LAR) was noted in 19/45 (42, 2 %) at a median age of 20.3 months (IQR 12.16 – 22.38). Of the 45 patients with ACR, 14/45 (31.1%) developed PCRR. PCRR was a form of LAR in the study which developed at a median time of 20.6 months (IQR 7.5 – 28). Significant risk factors for PCRR included increased frequency and severity of ACR episodes (p=0.001). PCRR was significantly associated with autoimmune seropositivity (p<0.001) and Chronic Rejection (CR) (p=0.006) but not patient survival (p=0.611). CR occurred in 10/45 (22.2%) of patients and was significantly associated with the number (P =0.023) and severity (P = 0.043) of rejection episodes and the presence of biliary strictures (P=0.010). Central perivenulitis was a significant pathological feature of PCRR (P <0.001) and CR (P=0.005) in the study. Cytomegalovirus (P=0,004), MLVI variance (P=0.025) and severity of ACR episodes (P=0.013) were associated with poor patient survival in multivariate analysis.
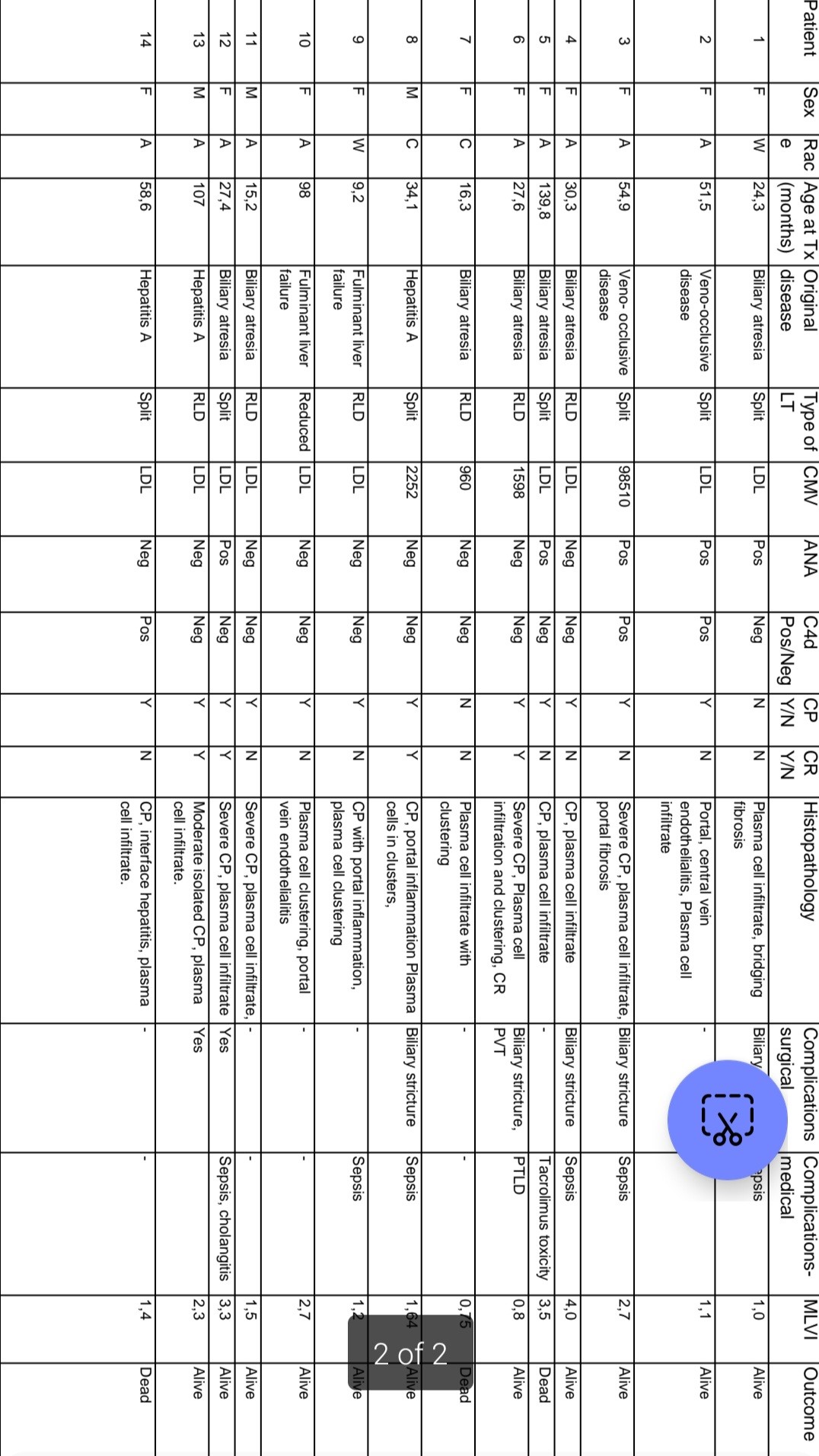
Conclusion: Increased frequency and severity of ACR episodes attributed to poor adherence to immunosuppression were associated with PCRR in our cohort of PLT recipients. CP is a hallmark histopathological feature in liver biopsies of patients with PCRR. The development of PCRR was associated with chronic rejection and graft failure, in PLT recipients in our cohort of patients.
[1] De novo autoimmune hepatitis; paediatric liver transplantation, plasma cell hepatitis; Plasma cell–rich rejection, acute cellular rejection, chronic rejection, antibody mediated rejection
