Randomized phase 2 trial of felzartamab in humoral transplant rejection
Katharina Mayer1, Eva Schrezenmeier2, Matthias Diebold1,3, Philip F Halloran4, Martina Schatzl1, Alexander Kainz1, Farsad Eskandary1, Konstantin Doberer1, Uptdal D Patel5, Jaideep S Dudani5, Heinz Regele6, Nicolas Kozakowski6, Johannes Kläger6, Kerstin Amann7, Elisabeth Puchhammer-Stöckl8, Hannes Vietzen8, Julia Beck9, Ekkehard Schütz9, Aylin Akifova2, Bilgin Osmanodja2, Fabian Halleck2, Bernd Jilma10, Klemens Budde2, Georg A Böhmig1.
1Division of Nephrology and Dialysis, Department of Medicine III, Medical University of Vienna, Vienna, Austria; 2Department of Nephrology, Charité Universitätsmedizin Berlin, Berlin, Germany; 3Clinic for Transplantation Immunology and Nephrology, University Hospital Basel, Basel, Switzerland; 4Alberta Transplant Applied Genomics Centre, University of Alberta, Edmonton, AB, Canada; 5Human Immunology Biosciences, Inc. (HI-Bio), South San Francisco, CA, United States; 6Department of Pathology, Medical University of Vienna, Vienna, Austria; 7Department of Pathology, University of Erlangen-Nürnberg, Erlangen, Germany; 8Center of Virology, Medical University of Vienna, Vienna, Austria; 9Chronix Biomedical GmbH, Göttingen, Germany; 10Department of Clinical Pharmacology, Medical University of Vienna, Vienna, Austria
Felzartamab Study Group.
Background: Antibody-mediated rejection (ABMR) is a leading cause of renal allograft failure. Targeting CD38 to inhibit alloantibody- and natural killer (NK) cell−driven injury may be a therapeutic option.
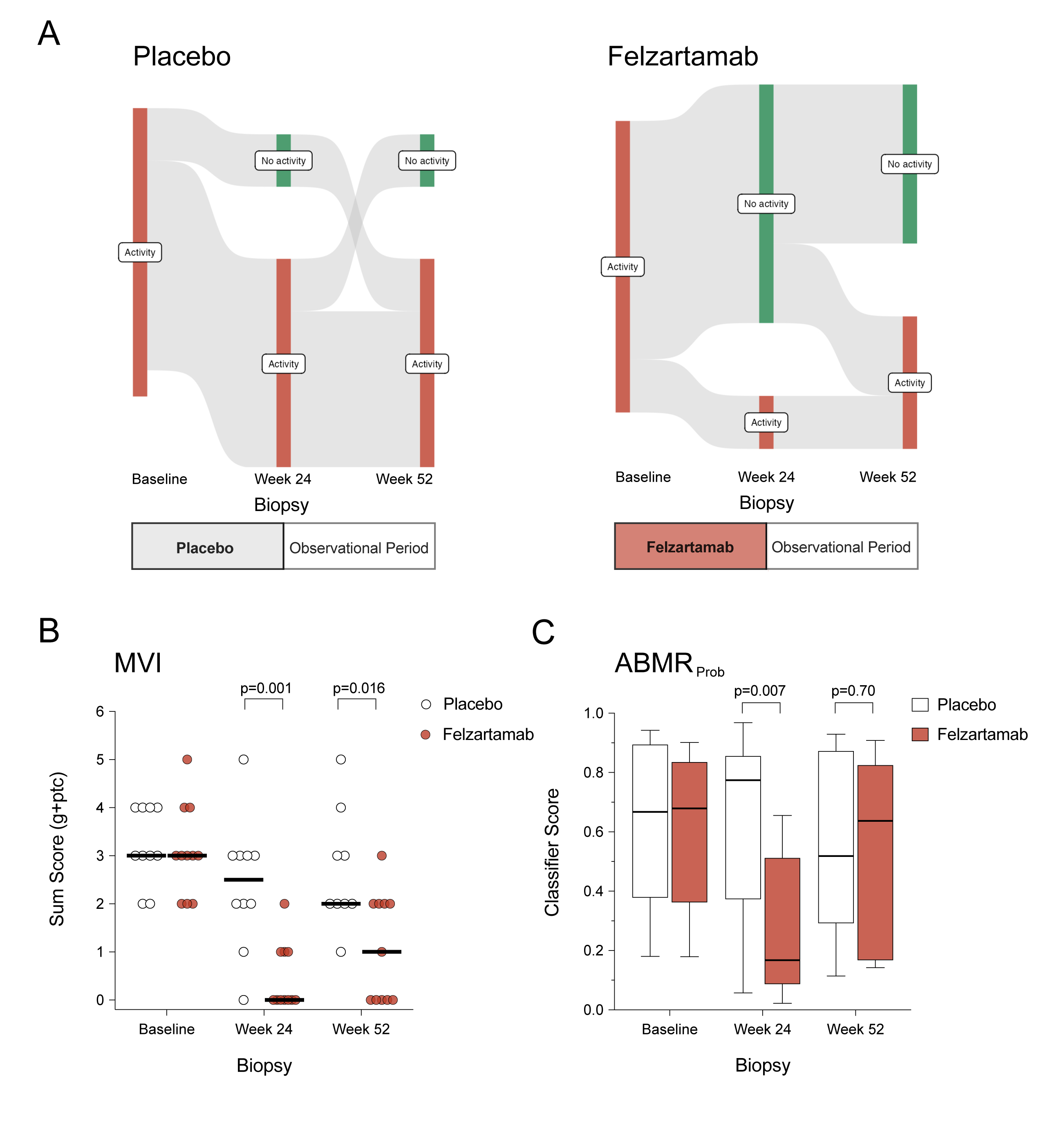
Methods: In a double-blind, placebo-controlled phase 2 trial, patients with ABMR ≥180 days post-transplant were randomized 1:1 to receive nine infusions of the CD38 monoclonal antibody felzartamab (16 mg/kg) or placebo over 6 months, followed by a 6-month observational period. The primary outcome was felzartamab safety and tolerability. Secondary outcomes included 24- and 52-week renal biopsies, donor-specific antibody (DSA) levels, peripheral NK cell counts, and donor-derived cell-free DNA (dd-cfDNA).
Results: Of 22 patients randomized (felzartamab, n=11; placebo, n=11), eight had mild to moderate infusion reactions (felzartamab, n=8; placebo, n=0) and five had serious adverse events (felzartamab, n=1; placebo, n=4). One patient who received placebo had graft loss. After week 24, resolution of morphologic ABMR activity was more frequent with felzartamab (9 of 11 [81.8%]) versus placebo (2 of 10 [20%]; P=0.009). Felzartamab decreased median (interquartile range) microvascular inflammation scores (0 [0−1] versus 2.5 [2−3]; P=0.001), molecular ABMR activity (ABMRProb: 0.17 [0.09−0.51] versus 0.77 [0.37−0.86]; P=0.007), CD16bright NK cell counts (16 [8−41] versus 54 [38-170] cells/ml; P=0.004), and dd-cfDNA (0.31 [0.21−0.49] versus 0.82 [0.34−2.90)%]; P=0.036). DSA changed minimally. At week 52, ABMR activity recurrence was observed in 3 of 9 felzartamab responders, with molecular activity and biomarker levels increasing towards baseline.
Conclusion: Felzartamab exhibited favorable safety and efficacy, underscoring its potential as a novel therapeutic option in ABMR.
[1] kidney transplantation
[2] antibody-mediated rejection
[3] CD38
[4] NK cells
[5] rejection therapy
