Tenofovir versus entecavir on the prognosis of hepatitis B-related hepatocellular carcinoma after surgical resection: A systematic review and meta-analysis
Bricia Almeida1, Juliana Almeida Oliveira2, Barkhá Vijendra3, Carolina Magalhães Costa4,5.
1University of Medical Sciences of Minas Gerais (FCMMG), Belo Horizonte, Brazil; 2Federal University of Minas Gerais (UFMG), Belo Horizonte, Brazil; 3National Institute of Health, Maputo Province, Marracuene district, Mozambique; 4Hepatology and Liver Transplantation, Hospital Sírio-Libanês, São Paulo, Brazil; 5Hepatology and Liver Transplantation, A. C. Camargo Cancer Center, São Paulo, Brazil
Introduction: Treatment with Nucleot(s)ide analogs, such as entecavir (ETV) and tenofovir (TDF), is first-line treatment for chronic hepatitis B. However, there is no consensus on the prognostic impact of each antiviral agent whenever hepatitis B virus (HBV) is related to the hepatocellular carcinoma (HCC) after curative liver resection.
Method: PubMed, Embase and Cochrane Trials database were systematically searched for randomized controlled trials (RCT) or cohorts that compared TDF to ETV for hepatitis B-related HCC after surgical resection. Review Manager 5 and R Studio were used as statistical tools and the DerSimonian and Laird random-effects model was applied for all outcomes. The entire process was performed by two authors and checked by a third author.
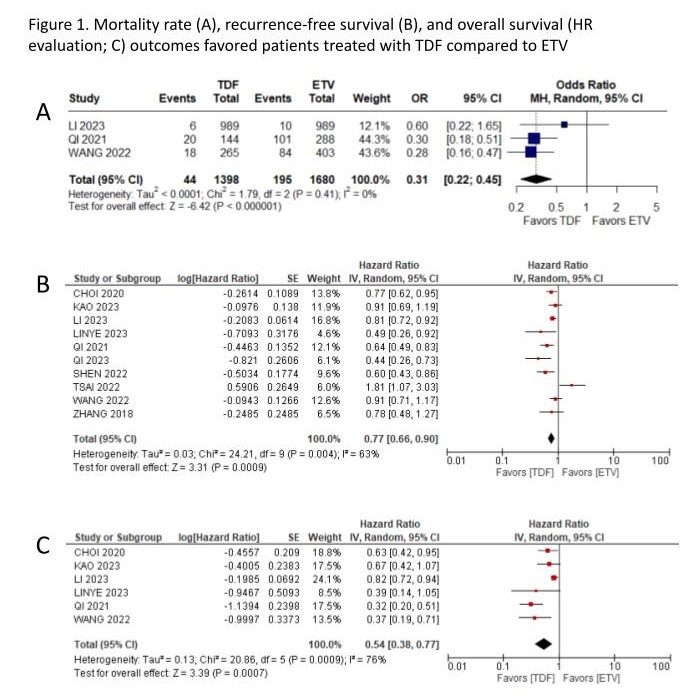
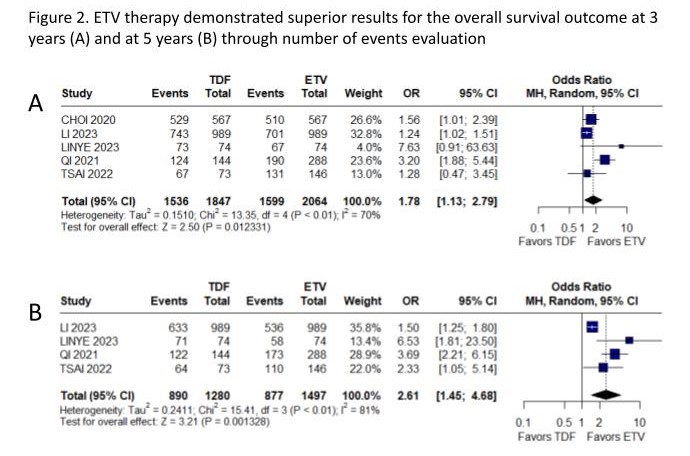
Results: The search yielded 435 results, of which 1 RCT and 10 cohort studies were included, encompassing 8036 patients, among whom 2819 (35%) received TDF. All available outcomes were assessed. The mortality rate (OR 0.31; 95% CI 0.22-0.45; p<0.01; Figure 1A), recurrence-free survival (RFS) (HR 0.77; 95% CI 0.66-0.90; p<0.01; Figure 1B), and overall survival, evaluated through Hazard Ratio (HR 0.54; 95% CI 0.38-0.77; p<0.01; Figure 1C), favored patients treated with TDF compared to ETV. Notably, when assessed through number of events, ETV therapy demonstrated superior results for the overall survival outcome at 3 years (OR 1.78; 95% CI 1.13-2.79; P<0.01, figure 2A) and at 5 years (OR 2.61; 95% CI 1.45-4.68; P<0.01, figure 2B). The variability in follow-up durations, spanning from 18 months (1.5 years) to 91 months (7.6 years), may have had an impact on the overall survival outcome findings. Considering RFS at 1 year (OR 1.04; 95% CI 0.46-2.34; p=0.93), 3 years (OR 126; 95% CI 0.69-2.28; p=0.45) and 5 years (OR 1.23; 95% CI 0.61-2.50; p=0.57), no significant difference was observed between groups. Neither the early (HR 1.06; 95% CI 0.74-1.54; p=0.74) and late RFS (HR 0.89; 95% CI 0.46-1.70; p=0.72), nor overall survival at 1 year (OR 1.97; 95% CI 0.79-4.89; p=0.14) demonstrated significance.
Conclusion: These findings suggest that TDF might exhibit superior efficacy compared to ETV as a therapeutic approach on HBV-related HCC subsequent to curative liver resection. Further investigations are necessary to evaluate overall survival rates and potential treatment-related side effects among this particular group of patients.
We would like to thank Rhanderson Cardoso and the Meta-analysis Academy Team for all support during the statistical analysis process. .
[1] meta-analysis
[2] hepatitis B
[3] hepatocellular carcinoma
[4] antiviral agents
