
Evaluation of donor-derived cell-free DNA (dd-cfDNA) levels in primary graft dysfunction (PGD) after lung transplantation
Jeany P. Villamizar1,2, Juan C. Fernandez1,2, Andres Pelaez1,2, Renata Ponsirenas4, Guil Rozenbaum1,2, Mauricio Tellez1,2, Sama Al-Bayati1,2, Suresh Manickavel1,2, Daniel Buitrago1,3, Juan C. Salgado1,2.
1Miami Transplant Intitute, Jackson Health System, Miami, FL, United States; 2Division of Pulmonary and Critical Care, Miller School of Medicine Miami, University of Miami, Miami, FL, United States; 3Division of Thoracic Surgery and Lung Transplant, Miller School of Medicine Miami, University of Miami, Miami, FL, United States; 4Medical Affairs, CareDx, Inc, Brisbane, CA, United States
Introduction: Primary Graft Dysfunction (PGD) is a common complication after lung transplantation that occurs within 72 hours of surgery and affects about 30% of the lung transplant population, being a major cause of death post-transplant. The pathophysiology of PGD is complex and believed to result from a combination of insults that occur during the lung procurement, storage, and implantation processes. Given the considerable morbidity and mortality associated with PGD, there is a need for risk stratification that could enable more intensive monitoring and care of these high-risk patients. dd-cfDNA levels in the months following transplantation predict long-term outcomes, including CLAD. Additionally, dd-cfDNA levels can provide insights into the degree of early allograft injury. Here, we evaluated the levels of dd-cfDNA according to the time post-transplant and PGD occurrence in a population under dd-cfDNA surveillance for allograft injury.
Methods: This was a retrospective evaluation of all only lung transplant recipients transplanted between Jan/2023 and Jan/2024 in surveillance with dd-cfDNA. Our surveillance protocol includes dd-cfDNA testing monthly post-discharge. PGD status was evaluated at 72 hours post-transplant and graded based on ISHLT grading. We compared the time to the first test evaluation between patients with PGD degree 3, PGD 1-2, and no PGD. Then, we compared the levels of dd-cfDNA in the first three months post-transplant between each group.
Results: We included 58 lung transplant recipients. Of those, 44 (75.9%) patients had no graft dysfunction, 3 (5.1%) patients had PGD 1, 4 (6.9%) had PGD 2, and 7 (12.1%) had PGD 3 at 72 hours. The mean time to 1st test was 53.1 (±37.52) days for the whole population; patients without PGD had significantly earlier draws than patients with PGD of any degree (Figure 1).
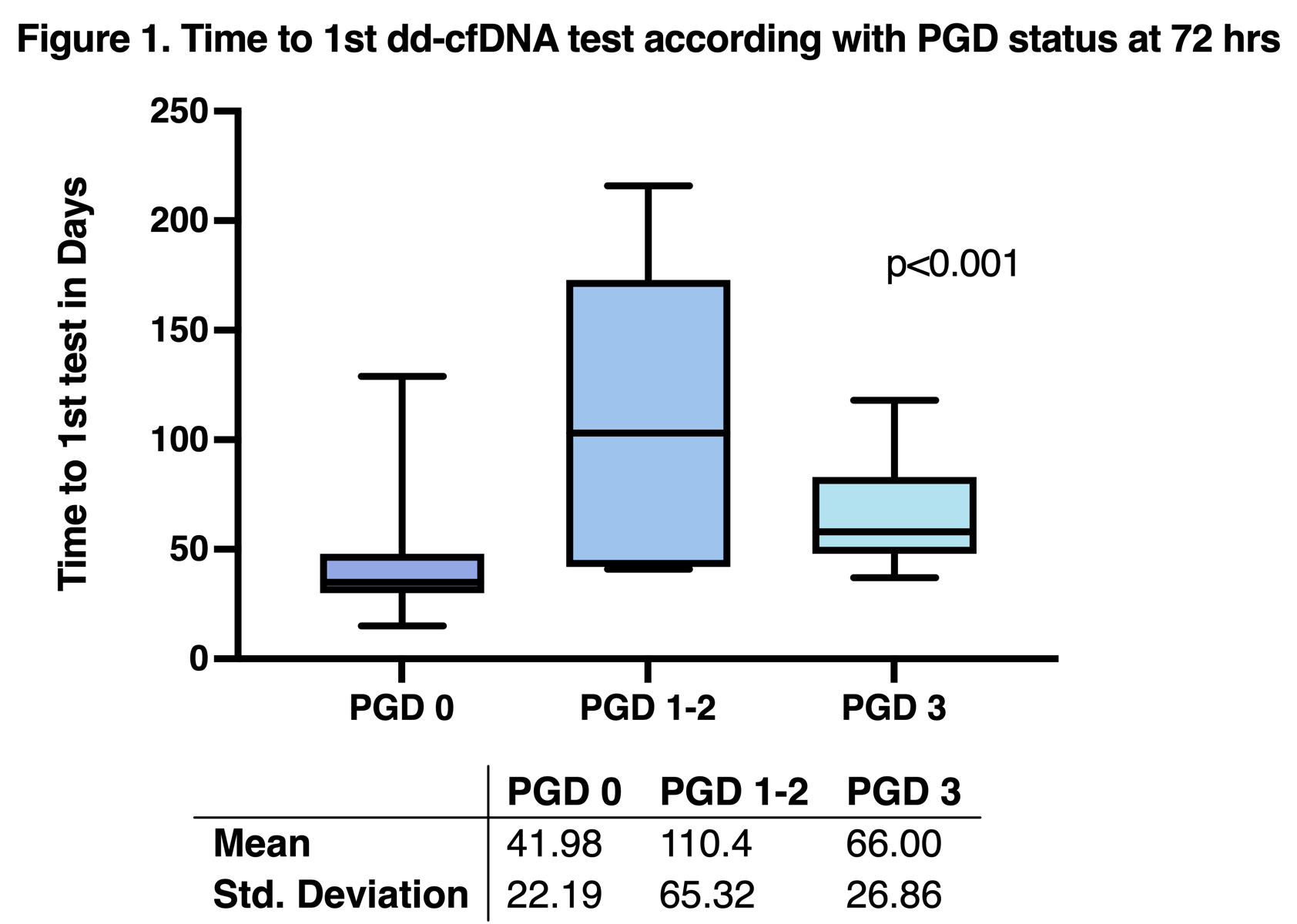
The dd-cfDNA median for the whole population was 0.78% (0.08-5.7%). Patients without PGD had higher levels of dd-cfDNA with a median of 0.94% (0.16-5.7%) compared with PGD 1-2 median of 0.28% (0.08-1.6%), PGD 3 median of 0.56% (0.33-1.9%). The dd-cfDNA trajectory of each group is depicted in Figure 2.
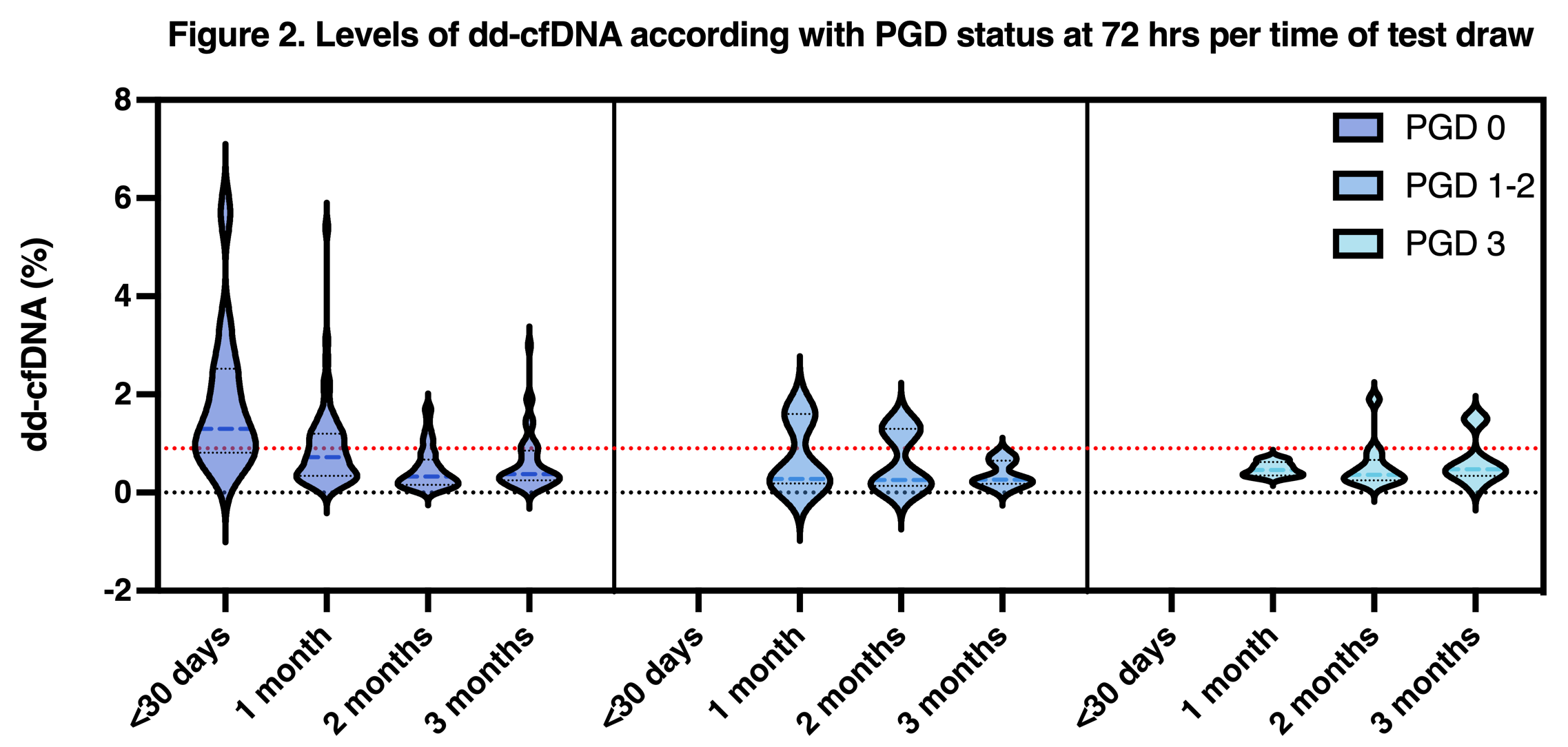
Patients with any degree of PGD didn’t have tests within the first 30 days of transplant; before completing 30 days, dd-cfDNA was higher for the patients with no PGD median dd-cfDNA of 1.3% (0.39-5.7%) compared with 0.72% (0.09-5.4%) from 30-60 days, and 0.33% (0.06-1.7%) 60-90 days, and 0.38% (0.06-3.0%) from 90-120 days post-transplant.
Conclusion: Patients without PGD had earlier tests with less than 30 days post-transplant, contributing to elevated levels of dd-cfDNA. The population with PGD was small, but there didn’t seem to be a trend towards higher levels of dd-cfDNA. More studies are needed to evaluate immune events correlations with these specific elevations.
[1] Lung Transplant, Primary Graft Dysfunction, Surveillance, Donor-Derived Cell-Free DNA
