The indication of liver transplantation for hepatocellular carcinoma in post-direct acting antivirals era in Japan
Ryoichi Goto1, Tsuyoshi Shimamura2, Tomoaki Nakajima3, Koji Ogawa4, Kazunari Tanaka5, Takao Shimizu5, Ryosuke Minami5, Takeshi Matsui5, Noriyuki Akutsu6, Shigeru Sasaki6, Ryo Kanazawa1, Norio Kawamura1, Masaaki Watanabe7, Ken Aiyama1, Akihisa Nagatsu7, Tatsuhiko Kakisaka1, Akinobu Taketomi1.
1Dept. of Gastroenterological Surgery 1, Hokkaido University Graduate School of Medicine, Sapporo, Japan; 2Division of Organ Transplantation, Hokkaido University Hospital, Sapporo, Japan; 3Dept. of Hepatology, Sapporo Kosei General Hospital, Sapporo, Japan; 4Dept. of Gastroenterology and Hepatology, Hokkaido University Graduate School of Medicine, Sapporo, Japan; 5Center for Gastroenterology, Teine Keijinkai Hospital, Sapporo, Japan; 6Dept. of Gastroenterology and Hepatology, Sapporo Medical University School of Medicine, Sapporo, Japan; 7Dept. of Transplant Surgery, Hokkaido University Graduate School of Medicine, Sapporo, Japan
Background: Recent development of treatment for hepatocellular carcinoma (HCC) and HCV viral infection in chronic liver failure may change the indication of liver transplantation (LT). The newly LT criteria for HCC, 5-5-500 rules; tumor size 5cm≥, tumor number 5≥, AFP 500≥, has been implemented in Japan since 2019 Aug. In addition, HCCs based on Child-Pugh (C-P) B and C but not C-P A were covered by national insurance and allowed wait-list registration for deceased donor LT in Japan. The aim of this study was to evaluate the national rule of LT for HCC in Japan.
Methods: The clinical data from adult 294 patients (PTs) who detected primary HCC between Jan. 2013 and Dec. 2017 were collected from multicenter in Sapporo, Japan. The median age was 59.7 (23.8-65.9) which was recognized as eligible for LT in Japan. Five were excluded due to insufficient data.
Results: Of 289 PTs with HCC, C-P A, B, and C were 215 (74.0%), 52 (18%) and 23 (8%), respectively. The HCCs within criteria of Milan and 5-5-500 rule were 179 (61.9%) and 185 (64.0%), respectively. The 1- and 5-year overall survival rates for patient with HCC within Japan criteria (5-5-500 and Milan) were 100% and 88.1% in C-P A, 95.8% and 35.2% in C-P B, 72.9% and 0% in C-P C when LT has not been performed (Figure 1).
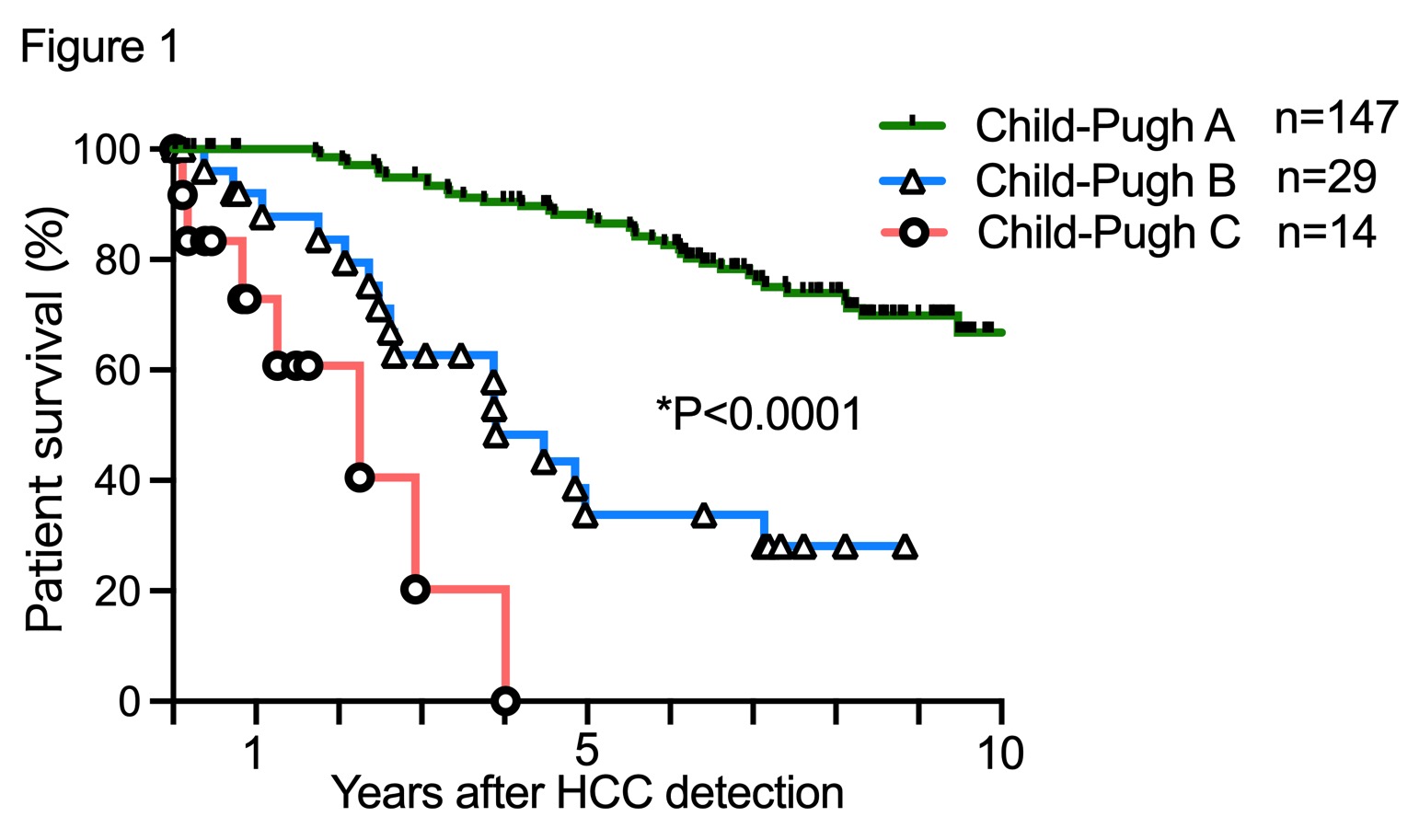
Meanwhile, TNM stage (P=0.0001, HR=3.93), HCC number (P=0.001, HR=3.01), C-P score (P=0.0011, HR=2.97), the albumin-bilirubin (ALBI) score (P=0.0015, HR=2.83), DCP (P=0.015, HR=1.821), Fib4 index (P=0.022, HR=1.66) and HCC size (P=0.021, HR=1.688) were significant prognostic risk factors in PTs with HCC within Japan criteria in C-P A. In particular, the ALBI-T score which calculated with ALBI grade and TNM staging, well-stratified the PTs in C-P A (Figure 2).
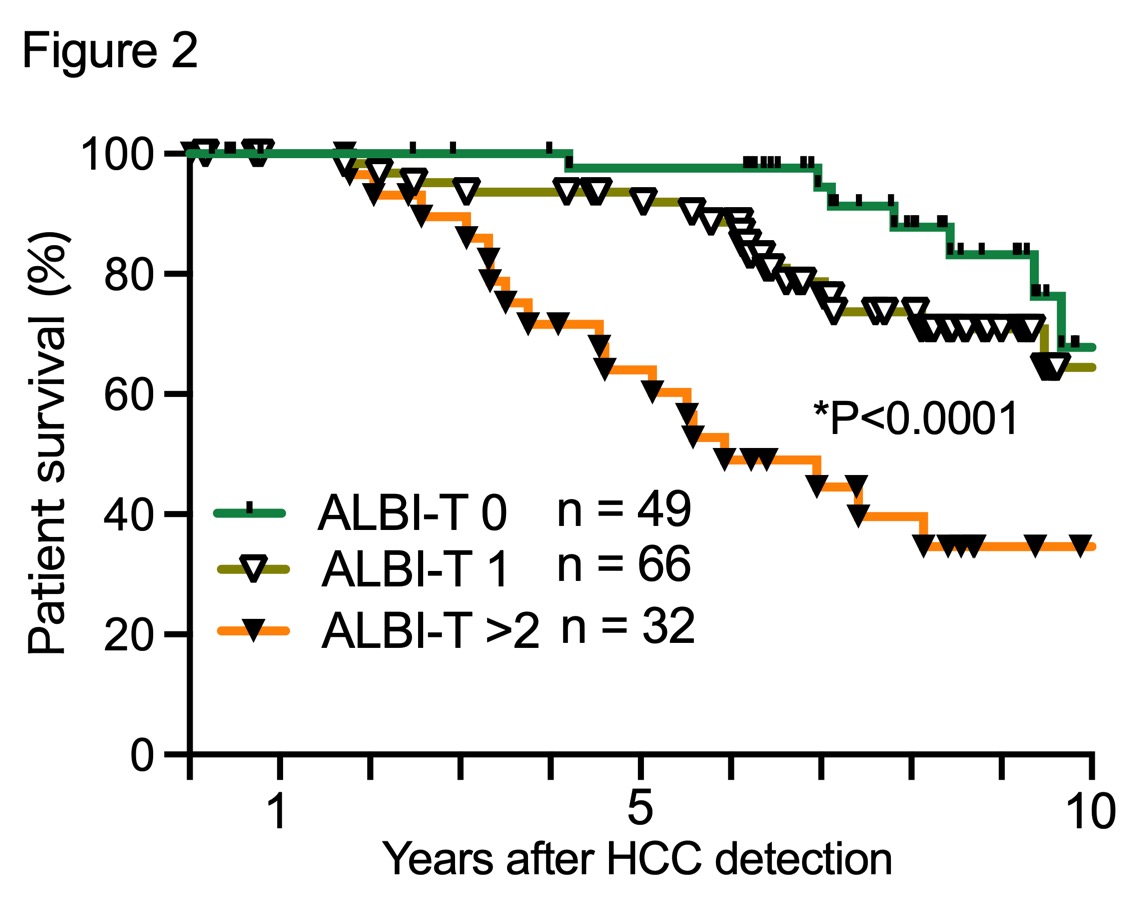
Conclusion: The Japanese national data showed that 5-year survival rates following living and deceased donor LT for PTs with HCCs were 70.7% and 78.6%, respectively. Thus, HCC within Japan criteria based on C-P B and C was absolutely recommended for LT. Further, the PTs with ALBI-T score of 2 or higher in C-P A should be considered for LT.
