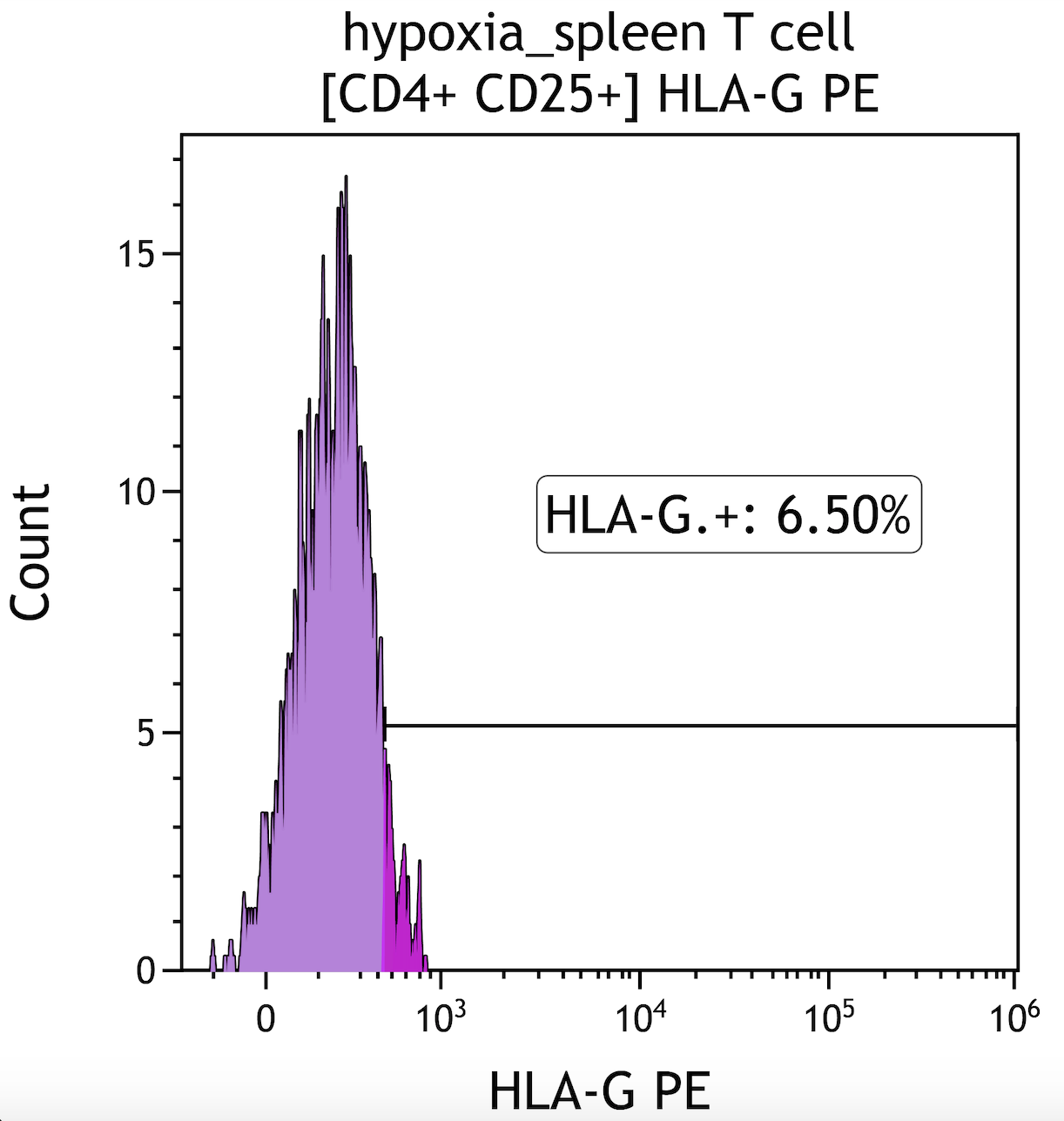
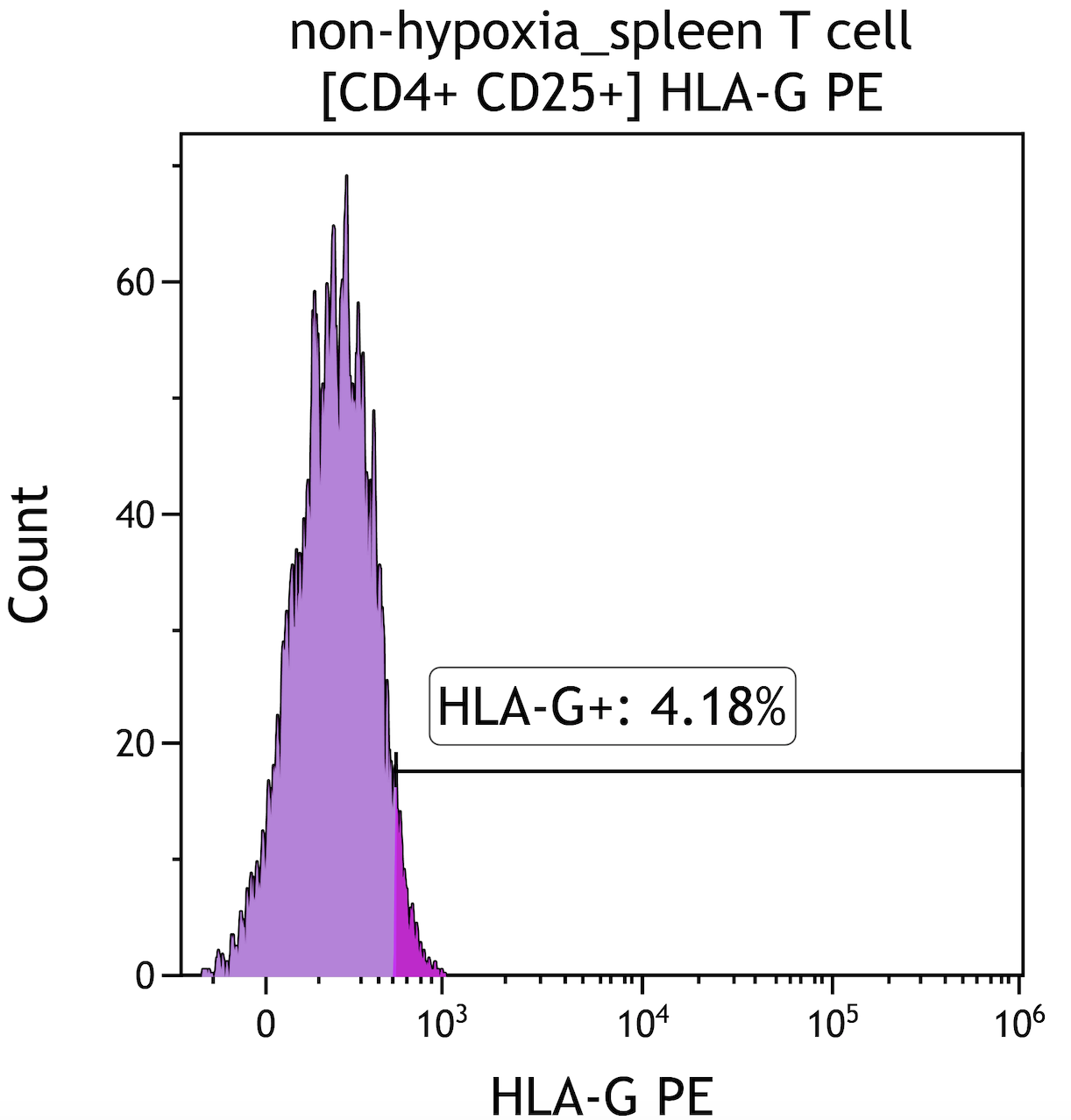
Ischemia change HLA-G expression in peripheral blood and spleen T lypmphocyte subsets
Miray Kavuzlu1, Bilkay Basturk1,2, Kenan Caliskan3, Atilla Sezgin4, Sedat Yildirim3, Mehmet A. Haberal3.
1Tissue Typing and Transplantation Immunology Laboratory, Baskent University, Ankara, Turkey; 2Department of Immunology, Baskent University, Ankara, Turkey; 3Department of General Surgery, Division of Transplantation, Baskent University, Ankara, Turkey; 4Department of Cardiovascular Surgery , Baskent University, Ankara, Turkey
Introduction: Ischemia-reperfusion injury is an event involving graft tissue. Human Leukocyte Antigen-G (HLA-G) molecules are non-classic class Ib antigens and characterized by tolerogenic and immune inhibitory functions. HLA-G expression is up regulated in pathological conditions such as transplantation. Main source of HLA-G expression is CD4+CD25high Treg cells and CD8+ T cells.
The aim of the study was to investigate the expression change of the HLA-G molecule in both spleen and peripheral blood follicular Treg and CD8+ T cells in hypoxic conditions.
Method: In this experimental study, whole blood and human spleen samples from 4 volunteers were used in this study. Samples were obtained from cadaveric organ donors at Başkent University Cardiovaskular Surgery at Ankara Hospital and from patients General Surgery at Başkent University Adana Hospital. The remaining part of the spleen samples approximately 2x2 cm2 in size, taken from brain-dead donor for cross-matching studies, were used after routine studies. Lymphocytes were isolated from the samples by density gradient centrifugation using Lymphoprep (Stemcell, Germany). T and B cells from the isolated lymphocyte were purified by negative selection magnetic beads (Stemcell, Germany). The purity of isolated T and B cells were determined by flow cytometry with anti-CD3-ECD and anti-CD19-FITC antibodies, and the purity over 95% were used. The cell surface markers were analayzed using flow cytometry (Navios, BC) before and after hypoxia. In the experimental study, a hypoxic enviroment was used to mimic the ischemic condition and cells were incubated an anaerobic incubator for four hours. Anti-CD3-ECD, anti-CD4-FITC, anti-CD8-FITC, anti-CD25-PC5, anti-CD45-PC5 and anti-HLA-G-PE (eBioscience, USA) antibodies were used for T cell surface staining, and anti-CD19-FITC, anti-HLA-DR-PC7 and anti-HLA-G-PE antibodies were used for B cell staining. 10x104 cells/µL for spleen T and B cells, 5x104 cells/µL for PBMC T cells and 10x103 cells/µL for B cells were used. All of these studies were performed for spleen and whole blood samples from 4 volunteers.
Results: Our study results showed that both peripheral blood and spleen follicular Treg and CD8+T lymphocytes increased HLA-G expression under hypoxic conditions (Fig 1, Fig 2). In T cells isolated from both spleen and PBMCs, CD4+ T and CD8+T cell ratio decreased in hypoxia. Both spleen and peripheral bood Treg cells (CD4+CD25+) and HLA-DR expression on B cells decreased in hypoxic environment.
Conclusion: HLA-G expression promoting tolerance and graft survival. This study is the first to show that HLA-G expression increases in spleen fTreg and CD8+T cells under hypoxic conditionsin humans. HLA-G could be an important parameter to follow up cellular rejection.