
Navigating immunological risk in highly-sensitized transplant candidates: Insights from DSA assessment and FCXM analysis
Thais Ferreira de Oliveira Freesz2, Carlos Stopato2, Cellen Bisi2, Juliana Bastos1, Renata Pereira de Souza4, Carlos Sergio Viggiani3, Hélcio Rodrigues3, David José Machado de Barros4, Elias David-Neto4, Gustavo Ferreira1.
1Transplant Unit, Santa Casa de Juiz de Fora, Juiz de Fora, Brazil; 2Immunology , Santa Casa de Juiz de Fora, Juiz de Fora, Brazil; 3Immunology , InCor, São Paulo, Brazil; 4Transplant Unit, Universidade de São Paulo, São Paulo, Brazil
Introduction: Transplanting highly sensitized patients poses a significant challenge, particularly in low-to-middle-income countries where desensitization protocols and rejection treatment have limited accessibility. Consequently, they tend to accumulate on waiting lists, facing low transplant probabilities and high mortality rates. It is of paramount importance to accurately assess the immunological risk of these patients, including the presence of donor-specific antibodies (DSA).
Method: We employed the adsorption-elution protocol based on the technique described by El-Awar in 2007, to determine if the DSA was derived from a spurious reaction from PRA Kit and assess the immunological risk in a highly-sensitized recipient presenting a weakly positive flow cytometry crossmatch (FCXM).
Results: EDSGDM, a female multiparous patient who has undergone multiple transfusions, was a candidate for paired transplantation. A potential donor, CDS, was identified, but there was a supposed DSA against the HLA-A30:04 antigen. Since the available Panel Reactive Antibody (PRA) kits did not include HLA-A30:04 antigen in the bead set, we considered the reaction against HLA-A*30:02 as a DSA, as these two molecules share an experimentally verified epitope (76EG).
FCXMs were conducted using two different sera from the recipient: one recent to the test date (28/02/2023) and the peak serum (13/12/2022). Both tests showed weak positivity only for B cells. Seven months later, new FCXMs were performed with two additional sera collected on 02/06/2023 and 11/09/2023. The results also indicated weak positivity only for B cells. 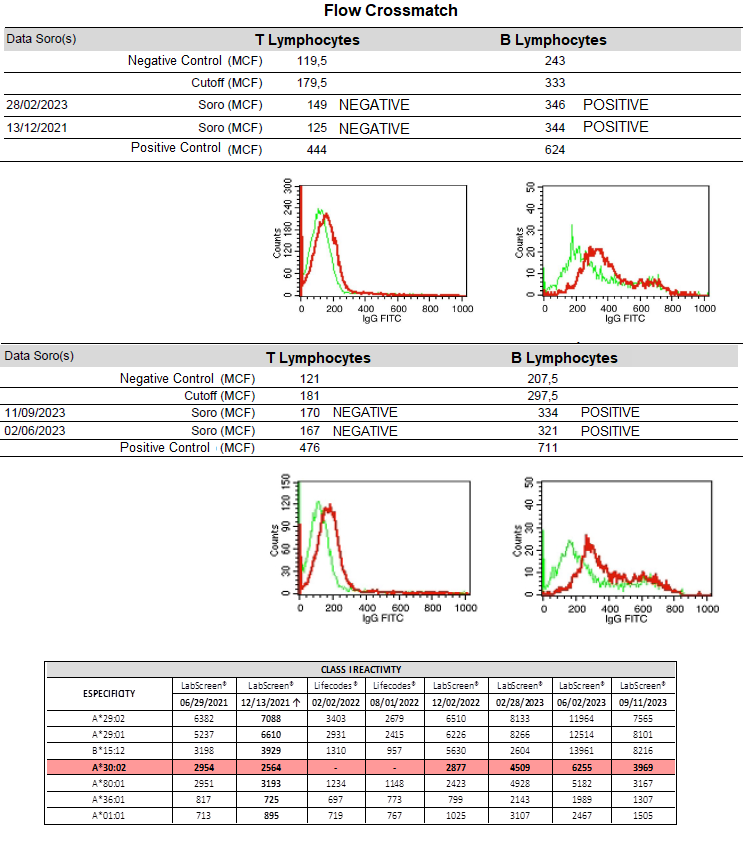
In light of this inquiry, we examined the Class I PRA results to determine if the reactivity against HLA-A30:02 could be a false positive. We employed an adsorption-ellution method using donor lymphocytes. The results revealed that the anti-A30:02 antibody was bound to the donor's lymphocytes and recovered in the eluted serum. Additionally, despite having a single target in the donor's cells (A30), anti-HLA A29, A80, A1 and A36 antibodies were also detected in the eluted serum, in addition to the anti-A30 antibody.
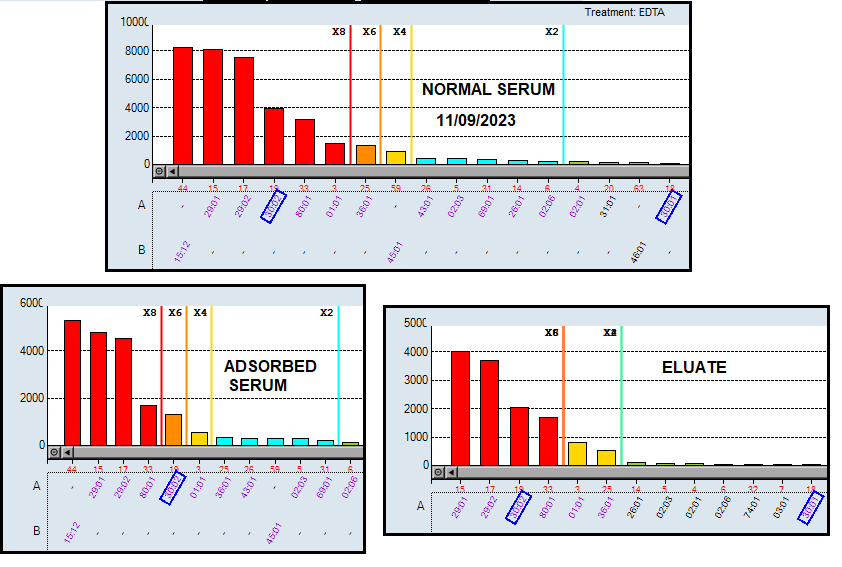
These findings suggest that the antibody against A*30:02 is a "true positive" and may account for the positivity observed in the FCXM. However, the question arises whether this result poses an impediment to carrying out the transplant.
Given the low positivity in FCXM, we decided to proceed with the transplant in February 2024. All tests were repeated using recent serum samples to ensure safety, yielding similar results. DSA levels and renal function have been consistently monitored and remain stable (MFI 2,300 and Cr 1.2).
Conclusion: In conclusion, the support of immunology laboratories with expertise in conducting and analyzing various types of immunological risk assessments is crucial for accurately evaluating high-immunological-risk patients and furnishing crucial insights for clinical decision-making.
[1] Graft Rejection
[2] kidney transplant
[3] Antiboty Mediated Rejection
