Correlating tacrolimus levels with dose formulation: implications for toxicity and renal graft rejection in a cohort of kidney transplant recipients with steroid-free maintenance immunosuppression
Natalia Gaitan-Torres1, Nicolás Lozano-Suarez2, Andrea Garcia-Lopez2, Andrea Gomez-Montero2, Fernando Giron-Luque3.
1Department of Transplant Pharmacology, Colombiana de Trasplantes, Bogota, Colombia; 2Department of Transplant Research, Colombiana de Trasplantes, Bogota, Colombia; 3Department of Transplant Surgery, Colombiana de Trasplantes, Bogota, Colombia
Introduction: Chronic kidney disease (CKD) affects 10% of the worldwide population, and renal transplantation stands out as the optimal treatment for advanced stages. Post-transplant care involves immunosuppressive therapy, primarily utilizing tacrolimus, to prevent graft rejection and failure. This study analyzes the correlation between tacrolimus blood levels, dosing, toxicity, and graft rejection among renal transplant recipients with Steroid-free maintenance immunosuppression in Colombia.
Methodology: We conducted an observational, analytical, retrospective cohort study on kidney transplant patients followed at Colombiana de Trasplantes from July 2019 to June 2022, including only kidney recipients who were receiving tacrolimus as part of their immunosuppressive maintenance therapy and who had completed at least one year of post-transplant follow-up. Data on tacrolimus levels, demographic information, and transplant outcomes were collected at four intervals post-transplant. Bivariate analysis and unadjusted logistic regression were used to analyze the correlation and association between tacrolimus blood levels and tacrolimus doses, rejection, and toxicity.
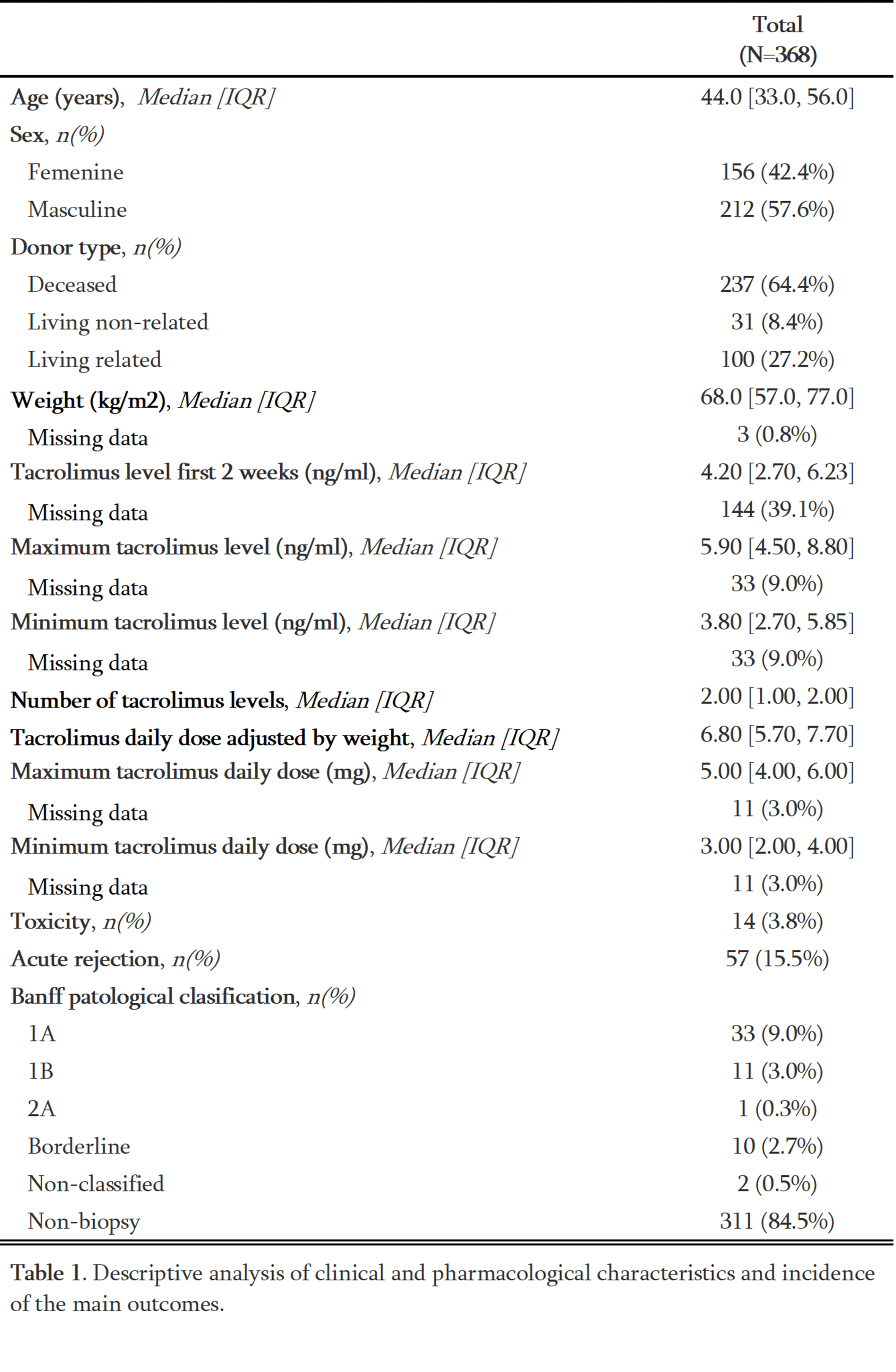
Results: The study included 368 patients. Tacrolimus levels were 4.98 ng/ml on average in the initial two weeks post-transplant; a weak correlation was found between variability of levels and dose (rho=0.31, p <0.001) but was not significant between tacrolimus doses and categorized blood levels in the first twelve months. Acute graft rejection occurred in 15.5% of patients, while only 3.8% experienced toxicity. No significant differences in rejection rates were observed with tacrolimus blood levels.
Conclusions: This study found no significant association between tacrolimus levels and graft rejection, toxicity or categorized tacrolimus blood levels within the first-year post-transplant. However, the importance of tacrolimus level monitoring is underscored by its variability influenced by dosing, adherence, and pharmacogenetics. Further research with a larger sample size is needed to improve our understanding of tacrolimus pharmacokinetics and encourage accurate reporting of toxicity in the Colombian population.

[1] Tacrolimus
[2] graft rejection
[3] toxicity
[4] Kidney Transplantation
[5] Clinical Pharmacology
