
CYP3A5 polymorphism in kidney transplantation: individualizing tacrolimus dosing
Amit S Pasari1,2, Manish R Balwani1,2, Charulata Bawankule1,2, Kapil Sejpal1, Prasad Gurjar1, Pranjal Kashiv1, Shubham Dubey1, Sunny Malde1, Sushrut Gupta1, Twinkle Pawar1.
1Nephrology, JNMC, Sawangi, Wardha, India; 2Nephrology, Saraswati Kidney Care Center, Nagpur, India
Introduction: Tacrolimus (TAC) is an integral part of maintenance immunosuppression in kidney transplant (KT) recipients. The genetic polymorphisms in CYP3A5 gene lead to differences in metabolism of TAC leading to underdosing or toxicity. Understanding the type of CYP3A5 polymorphism can help tailor the dose of TAC in KT recipients. Though routine evaluation of CYP3A5 gene polymorphism is not necessary, high prevalence of CYP3A5 polymorphism observed in our practice prompted us to evaluate the KT patients for presence of polymorphisms. In this study, we assessed the prevalence of polymorphisms and studied their association with TAC dose requirements and toxicities.
Methods: The electronic database at our transplant unit from central India was reviewed to include the KT patients who were assessed for CYP3A5 gene polymorphism. Patients with homozygous polymorphism (CYP3A5*3/*3) with substitution of G for A at position 6986 (6986 A>G), located within intron 3 of the CYP3A5 gene were considered non-expressers and those who carried at least one copy of A gene (CYP3A5*1/*3 or CYP3A5*1/*1) were considered as expressers. Polymorphism was diagnosed by polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) method. Trough TAC levels of 7 to 10 ng/ml and 3 to 7 ng/ml within first six or after six months after KT were considered as therapeutic levels. Concentration to dose ratio of TAC (Co/D) was assessed for individual patients. Rates of complications such as infections and new onset diabetes after transplantation (NODAT) were also assessed and compared.
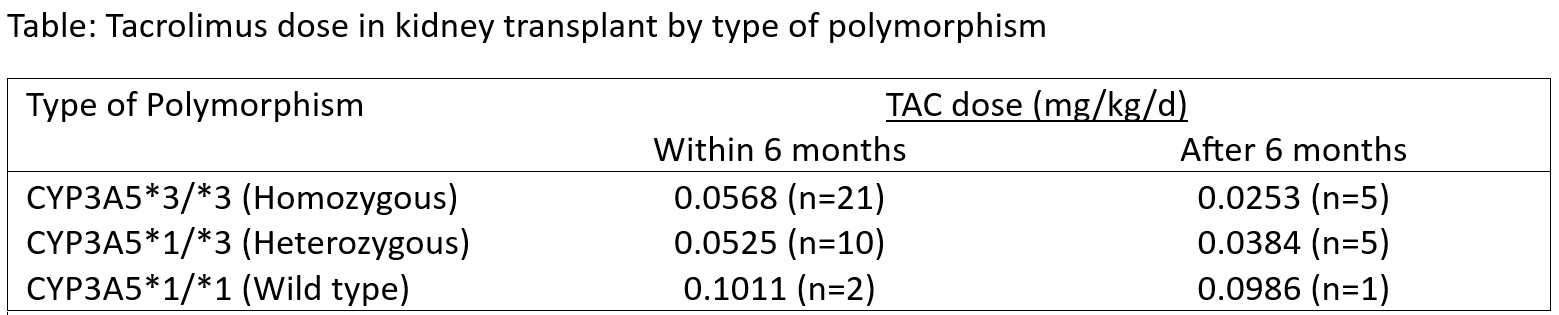
Results: Between January 2017 and February 2024, 71 patients underwent testing for CYP3A5 polymorphism. Among them, 41 (57.7%) and 30 (42.3%) were non-expressers and expressers respectively. A total of 54 (76.0%) patients had undergone KT. The mean age was 37.6±12.2 years and 42 (77.8%) were males. Among KT recipients, 33 (61.1%) and 21 (38.9%) were non-expressers and expressers, respectively. The diagnosis of polymorphism was pre-transplant in 29 (53.7%) cases. Nine cases did not receive TAC. TAC dose requirement was lower in non-expresser (Figure). The median Co/D was 114.4 ng/ml/mg/kg/d with higher value in non-expresser than expressers (129.5, [n=26] Vs. 105.9 [n=13] ng/ml/mg/kg/d respectively, p=0.435). Excluding nine cases of existing diabetes, NODAT occurred in 11 (25.6%) patients with nearly equal proportions in expressers and non-expressers (25% vs. 23.5% respectively, p=0.911). Urinary tract infection (27.8%), lower respiratory tract infection (11.1%), pulmonary tuberculosis (3.7%), and TAC related neurotoxicity (1.8%) were the complications noted with no statistical difference in two groups. Mortality occurred in two patients.
Conclusion: Understanding CYP3A5 polymorphism status can help achieve therapeutic TAC concentration with greater ease. Adjusting the dose based on specific polymorphism can help achieve the balance of efficacy and safety in KT recipients.
Dr Vijay Katekhaye. Miss Kajal Gajbhiye. Miss Shalini Patle.
[1] CYP3A5 polymorphism
[2] Kidney Transplantation
[3] Tacrolimus
[4] Personalized medicine
