The outcomes of immunosuppression modification in mycophenolate mofetil-related gastrointestinal mucosal injury in kidney transplant recipients
Abdullah Ansari1, Anupma Kaul1, Nandita Chaudhary2, Manas Behera1, Narayan Prasad1, Dharmendra Bhadauria1.
1Nephrology, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, India; 2Pathology, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, India
Objectives: Mycophenolate mofetil (MMF) causes gastrointestinal adverse effects in 45% of kidney transplant recipients, as enterocytes are partially dependent on the de-novo pathway of purine synthesis. The study aims to describe the outcomes of immunosuppression modification in symptomatic kidney transplant recipients having histopathological evidence of MMF-related toxicity.
Methods: A retrospective observational study from January 2009 to December 2018 at a tertiary care center in north India. All cases that had evidence of MMF-associated histopathological changes and underwent immunosuppression modification were included and studied.
Results: Among 122 recipients who underwent endoscopic biopsy, MMF-associated histopathological changes were observed in 102 (83.6%) cases. The indication of endoscopy was persistent or chronic diarrhea in 83.3% (n=85) and non-diarrheal causes in 16.7% (n=17) cases. The associated features were anemia (45.1%, n=46), weight loss (37.3%, n=38), and rectal bleeding (6.9%, n=7). The median duration was 57 (IQR 17-74) months post-transplant. The mean creatinine was 1.84 ± 0.91 mg/dl at the time of biopsy. All patients were on a triple immunosuppressive regimen, with either mycophenolate mofetil (81.4%, n=83) or enteric-coated mycophenolate sodium (16.7%, n=17). The comorbidities included diabetes (40.2%, n=41), tuberculosis (26.5%, n=27), CMV infection (37.3%, n=38), prior antirejection therapy (33.3%, n=34), and chronic renal allograft injury (46.1%, n=47). 2 cases had coexisting post-transplant lymphoproliferative disorder.
Despite conservative management and treatment of associated conditions, 61 (59.8%) patients with persistent symptoms required immunosuppressive drug modification. MMF was changed to enteric-coated mycophenolate sodium (ECMPS) in 20 cases, Azathioprine in 25 cases, and mTOR inhibitors in 4 cases, while MMF/ECMPS was discontinued completely in 12 cases. A significant number of patients (n=18, 29.5%) developed biopsy-proven rejection after drug modification (p=0.002). However, the difference in graft loss after immunosuppression modification was not significant (p=0.24). MMF was reintroduced in 6 patients, but two patients had diarrhea recurrence. During a median follow-up period of 30 months in the immunosuppression modification group, ten patients had chronic renal allograft injury (CRAI), including five graft losses; 17 patients died, and 19 patients were lost to follow-up.
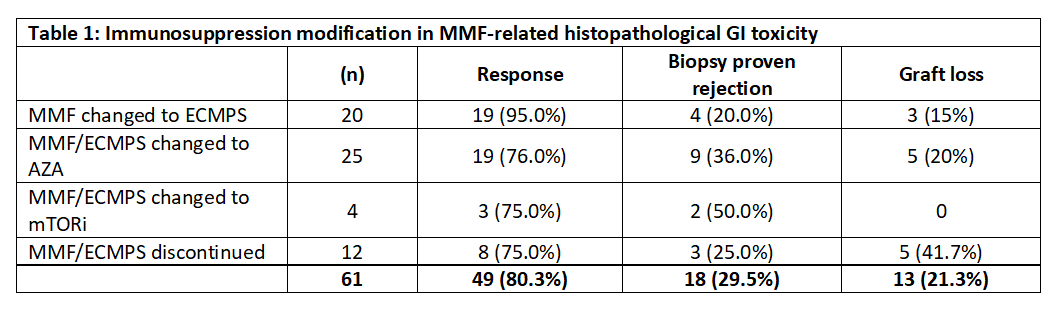
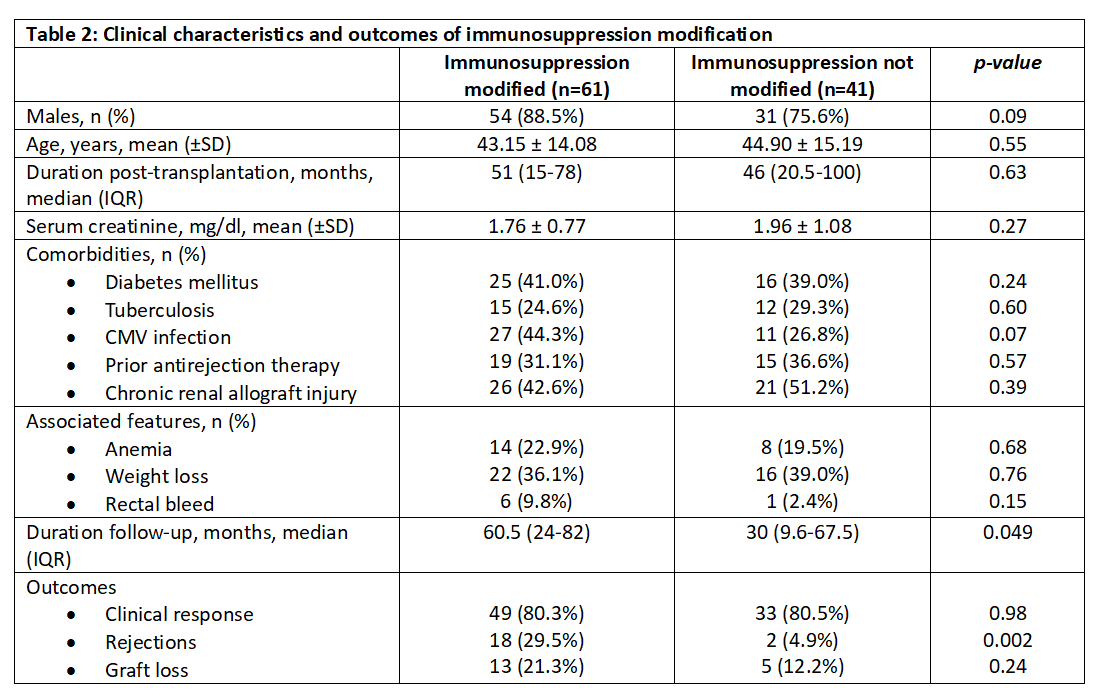
Conclusions: GI toxicity related to mycophenolate therapy is pretty prevalent in kidney transplant recipients. The severe symptomatic cases require immunosuppression modification, which can result in an increased risk of rejection and de-novo DSA formation.
None.
[1] kidney transplant
[2] mycophenolate mofetil
[3] gastrointestinal toxicity
