Association of non-HLA autoantibodies and CLAD following lung transplantation
Steven Hiho1,2, Bronwyn Levvey2, Gregory Snell2, Lucy Sullivan2,3, Glen Westall2.
1Victorian Transplantation and Immunogenetics Service, Australian Redcross Lifeblood, Melbourne, Australia; 2Lung Transplant Service, Department of Respiratory Medicine, Alfred Hospital and Monash University, Melbourne, Australia; 3South Australian Transplantation and Immunogenetics Service, Australian Redcross LifeBlood, Adelaide, Australia
Introduction: Chronic lung allograft dysfunction (CLAD) is the major cause of rejection following lung transplantation. Although Human leukocyte antibodies (HLA) are major factor leading to CLAD, recent literature in solid organ transplantation suggests non-HLA autoantibodies could have a role in adverse transplant outcomes. Here in a retrospective, single centre cohort we investigate the association with CLAD and presence of pre-transplant autoantibodies in lung transplant recipients.
Method: Serum from lung transplant recipients were retrospectively tested for non-HLA autoantibodies (Labscreen). CLAD was defined for all recipients and used for analysis. In total 68 recipients were tested 39 autoantibody targets, and MFI cut-offs were determined by using 1 standard deviation from mean of the No CLAD group for all analysis. Kaplan Meier with log ranked tests and ANOVA were used to investigate autoantibody reactivity between CLAD and No CLAD groups.
Results: Of the 68 recipients, positivity ranged from 0-18 targets, with an average of 4 positive targets. 14 recipients had positivity to ≥8 autoantibodies, of these 9 developed CLAD. With recipients having ≤7 positive autoantibodies there was an association with increased freedom from CLAD (p< 0.01) (Figure 1).
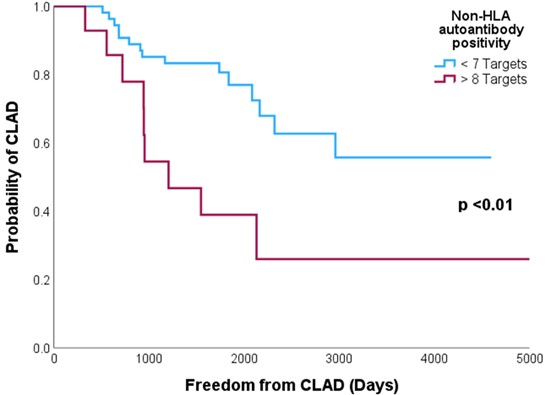
Conclusion: recipients with increased positivity to non-HLA autoantibodies pre-transplants demonstrate increased risk of CLAD and decreased freedom from CLAD. Further research with larger cohorts is needed to determine which autoantibody targets associated with outcomes, and to define best cut-offs to define reactivity.
We acknowledge The Lungitude Foundation and Australian Red Cross Lifeblood for their support with this study.
[1] Autoantibodies
[2] CLAD
[3] Non-HLA
