Lung uDCD protocol refinement in partnership with clinical, regulatory, and community stakeholders in New York City
Stephen Wall1,3,4,6, Jingzhi Xu3, Carolyn Sidoti3, Kenya Velez1, Luis Angel4, Stephanie Chang4,7, Justin Chan4,7, Brendan Parent5, Macey Levan3, Robert Montgomery2,4.
1Emergency Medicine, NYU Grossman School of Medicine, New York, NY, United States; 2Surgery, NYU Grossman School of Medicine, New York, NY, United States; 3Center for Surgical & Transplant Applied Research, NYU Grossman School of Medicine, New York, NY, United States; 4NYU Langone Transplant Institute, NYU Grossman School of Medicine, New York, NY, United States; 5Population Health Division of Medical Ethics, NYU Grossman School of Medicine, New York, NY, United States; 6Population Health Institute for Excellence in Health Equity, NYU Grossman School of Medicine, New York, NY, United States; 7Cardiothoracic Surgery, NYU Grossman School of Medicine, New York, NY, United States
Introduction: University of Toronto investigators evaluated a lung uDCD protocol that uses positive end-expiratory pressure (PEEP) and oxygen to preserve lungs noninvasively ~3 hours after death, but transplant yield after EVLP was 36% in part because organ donation authorization was required before in-situ preservation. Our research suggests the US public might permit noninvasive preservation without requiring prior permission to increase transplant yield. The objective was to refine the Toronto lung uDCD protocol for implementation in NYC.
Method: This action research study occurred from July 2022 to March 2024 with a consortium of NYU Langone Health and organ procurement organization (OPO) administrative leaders and clinical staff and community stakeholders from secular and religious organizations. Primary documents (meeting minutes, field notes) were systematically amassed and summarized into an action research matrix. Qualitative analysis of summary text was done with an iterative coding scheme based on the Consolidated Framework for Implementation Science. Stakeholder engagement occurred until thematic saturation was achieved.
Results: We held 17 focus groups with 85 participants, 2 clinical simulations with 18 participants, 10 town halls with community and religious organizations, 10 meetings with hospital and OPO leaders, and 30 informal communications totaling 69 encounters. Themes emerged representing individual perception, implementation climate, engagement, organizational capacity, and readiness for implementation. Barriers voiced included: 1) ethical/legal concerns from initiating preservation without requiring permission; 2) compressed time required to initiate preservation, converse with authorized decision-makers, and conduct clinical screening; 3) difficulty interpreting eligibility criteria; and 4) concerns about public acceptance.
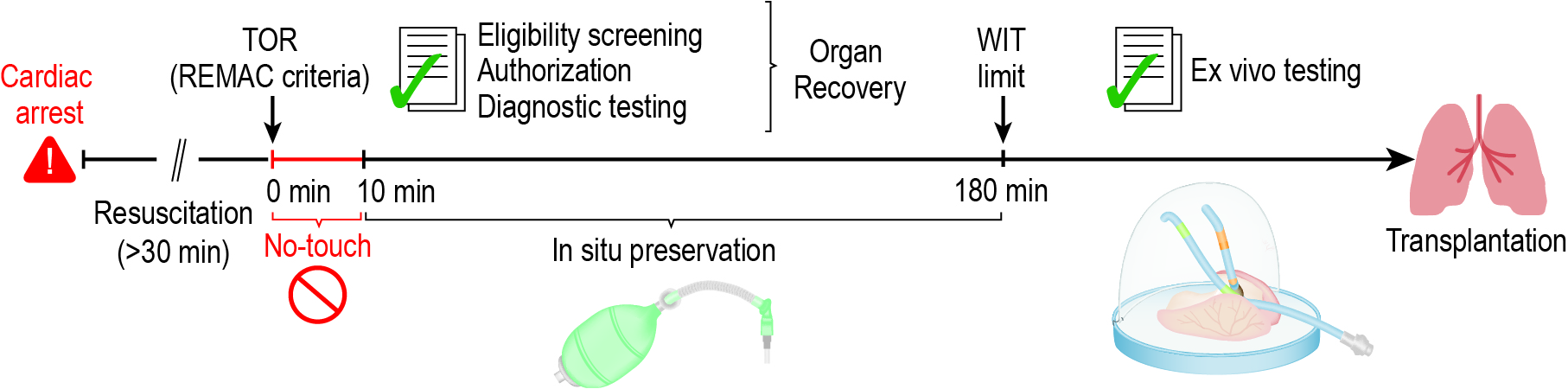
The lung uDCD protocol (Fig. 1) was refined to address barriers. After termination of resuscitation (TOR), bag-valve devices will remain attached to comply with the New York Anatomical Gift Act clause to not withdraw care until organ donation eligibility is ascertained. Additional hospital staff will serve as delegates of the OPO to provide 24/7 coverage to communicate with families about noninvasive lung preservation and request donation authorization. Clinical screening tests will occur rapidly within hospitals with courier services for offsite testing. A lung preservation specialist will be on call 24/7 for eligibility queries. An ethics board will review all case notes to determine ethical acceptability. Outcomes will be shared with an independent data and safety monitoring board to determine whether the program can continue.
Conclusion: The refined protocol initiates lung preservation without permission with strict regulatory oversight for up to 3 hours after death, is compliant with the US opt-in organ donation system, and is likely to be accepted by stakeholders who participated in its refinement.
Figure 1: Refined Lung uDCD Protocol
U.S. National Heart, Lung, and Blood Institute Grant Number R61HL156890. We thank wholeheartedly our collaborator, Marcelo Cypel MD MSc FACS FRCSC, who shared the University Health Network of Toronto Lung uDCD Protocol with our study team. Dr. Cypel will be included in the authorship for upcoming papers and will participate in the clinical safety and ethical evaluation of the refined protocol starting in July, 2024.
[1] Uncontrolled donation after circulatory death
[2] uncontrolled donation after cardiac death
[3] qualitative research
[4] action research
[5] lung preservation
[6] rapid organ recovery
