Donor-derived cell-free DNA is a valuable surveillance tool after single lung transplantation: Multi-center analysis
Ambalavanan Arunachalam1, Fatima Anjum2, Justin Rosenheck3, Reinaldo Rampolla4, Reda Girgis5, Howard J Huang6, Kathryn Crabtree7, Sarah McCormick7, Zhiji Zhang7, Cesar Escrig7, Sangeeta Bhorade7, David J Ross7.
1Division of Pulmonary and Critical Care, Northwestern University Feinberg School of Medicine, Chicago, IL, United States; 2Lewis Katz School of Medicine, Temple University, Philadelphia, PA, United States; 3Division of Pulmonary, Critical Care, and Sleep Medicine, The Ohio State University, Columbus, Columbus, OH, United States; 4Department of Cardiac Surgery, Cedars-Sinai Medical Center, Los Angeles, CA, United States; 5Corewell HealthCare, Grand Rapids, MI, United States; 6Houston Methodist Lung Transplant Center, Houston Methodist Hospital, Houston, TX, United States; 7Natera, Inc., Austin, TX, United States
Introduction: Donor-derived cell-free DNA (dd-cfDNA) is a validated biomarker for acute rejection (AR) and other types of graft injury after lung transplantation (LT). Despite a higher risk demographic, vulnerable to associated risks of protocol trans-bronchial biopsy (TBBx), limited dd-cfDNA studies specifically address single lung transplantation (SLT). Herein, we analyzed performance of dd-cfDNA in SLT patients from 6 academic LT centers who implement dd-cfDNA surveillance in standard of practice (SOP).
Method: dd-cfDNA (the Prospera™ Lung test; Natera, Austin, TX) results were analyzed with EMR clinical data review to assign an allograft status cohort: Acute Rejection (AR), STABLE, Infection (INFXN), “probable AR”, Chronic Lung Allograft Dysfunction (CLAD), or “OTHER”. Acute Lung Allograft Dysfunction (ALAD = AR+INFXN combined), was further analyzed. The bootstrapping approach was used to account for potential dependence between samples that came from the same patient. Dd-cfDNA results were corrected for SLT (2x) by the CLIA-lab algorithm. Cohorts analyzed for median (25- 75% IQR), comparisons by Mann-Whitney U-test with False Discovery Rate (FDR) correction (p<0.05), and AUC-ROC for AR vs Stable and ALAD vs Stable.
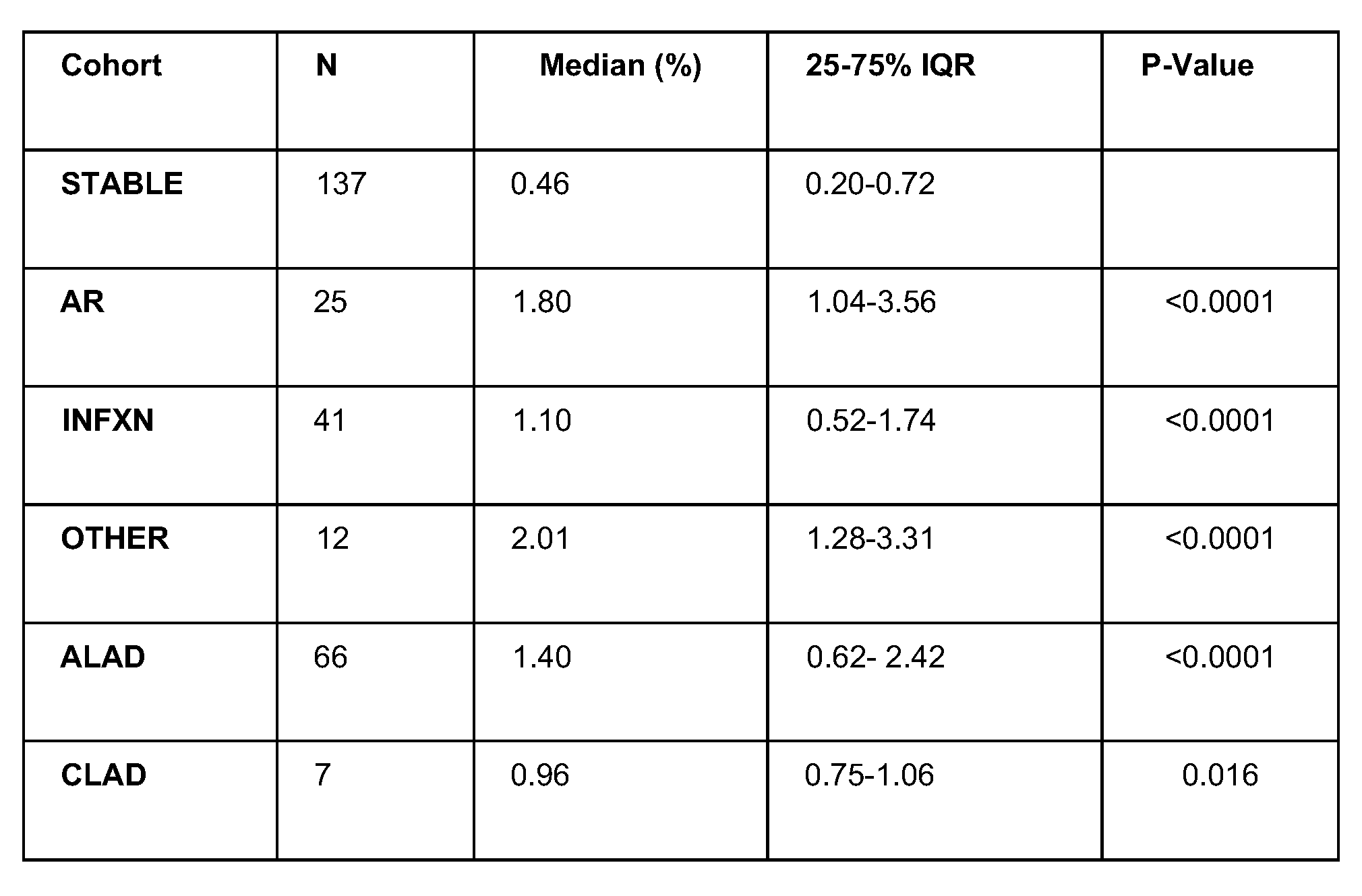
Results: Between 12/10/2021-10/27/2023 257 dd-cfDNA tests from 102 SLT patients were assigned and analyzed. Patient median age at SLT was 68.8y (64.1-71.5) and F:M 0.65. Time post-SLT to AR was 456.0 days (153-570; adjusted p=0.046), INFXN 264.0 days (129-503; p=0.266), CLAD 671.0 days (473-825; p=0.003), and ALAD 339.5 days (132-528; p=0.0504). vs STABLE 230.0 days (130-334). Analysis of laterality (R vs L) for AR vs. STABLE (p=0.821) was not different. In TABLE 1, dd-cfDNA fraction was elevated with AR (1.80%), INFXN (1.10%), CLAD (0.960%), and ALAD (1.40%) vs STABLE (0.46%; p<0.0001). For AR vs STABLE, AUC-ROC curve was 0.85 (0.72-0.95); applying a 1% dd-cfDNA threshold: sensitivity=77.8% (58.3-94.1), specificity=84.6% (76.0-91.9), PPV=38.3% (26.9-55.0), NPV=96.8% (94.2-99.2). For ALAD vs STABLE, AUC-ROC was 0.76 (0.67-0.84) with sensitivity=61.5%, specificity=84.7%, PPV=62.3%, NPV=84.3%.
Conclusion: Similar to reports for double LT, these multi-center data and real-world experience support clinical validity and utility of dd-cfDNA surveillance for SLT. The impact of biomarker surveillance on clinically meaningful outcomes should be forthcoming from robust, prospective clinical trials in progress.
[1] dd-cfDNA
[2] Lung Transplant
[3] Rejection
[4] Biomarker
[5] Single Lung Transplant
