
I'm a Pediatric Nephrologist, trained at the National University of Asuncion, Paraguay. I have completed a research stay at the JP Garrahan Pediatric Hospital in Buenos Aires, Argentina. I am deeply passionate about research in pediatric kidney transplantation and I am highly motivated to learn continually to contribute to improving the quality of life for transplant patients. My current focus is on data pertaining to children with PTLD and FSGS, with the aim of publishing these findings.
Lymphoproliferative disease in kidney transplanted children: What has happened over three decades? A single center experience from Argentina
Gabriela Gutièrrez1, Juan Ibáñez 1, Claudia Sarkis 2, Diana Di Pinto1, Veronica Solernou3, Daniela Borgnia4, Catrian Sotelo5, Marta L Monteverde1.
1Renal Transplant Section, Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; 2Infectious Diseases Unit, Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; 3Pathology Unit, Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; 4Virology Section, Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina; 5Oncology Unit, Hospital de Pediatría J.P. Garrahan, Buenos Aires, Argentina
Introduction: Post Transplant lymphoproliferative disorders (PTLD), the most common neoplasms in transplanted children are mostly associated with Epstein-Barr virus (EBV). The aims of our study were: 1. To describe, in a cohort of kidney transplant patients (KTx), cumulative incidence, incidence rate, time of onset (early <1 yr vs. late >1 yr), and clinical and histopathological characteristics of PTLD; 2. To compare patient and graft survival between those with and without PTLD and, in those EBV negative, association between peri-transplant administration of rituximab and primary EBV infection, EBV loads, and development of PTLD
Methods: Retrospective descriptive analysis including 1072 consecutive KTx recipients between Dec,1988 and Aug,2023. PTLD was diagnosed according to the WHO Criteria (2008/2016). To investigate association between rituximab and appearance of PTLD, we included EBV negative patients given rituximab. Statistical tests were performed using STATA version 18. Outcomes were measured with Kaplan-Meier models and overall strata comparisons were measured using log-rank tests. . For the analyses, P-values <0.05 were considered statistically significant.
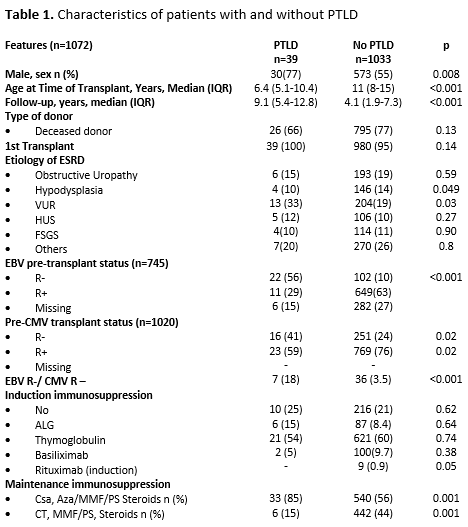
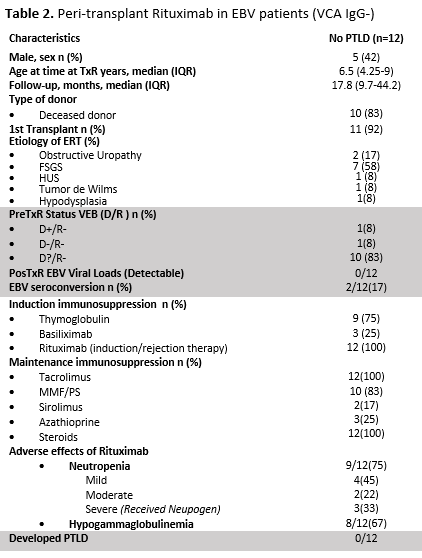
Results: PTLD was diagnosed in 39 patients (Table1). Cumulative incidence over the period was 3.64%. Before 2011 (Era1), when viral load was not monitored, it was 5,4% (n=34/627), while after (Era2) it dropped to 1.12% (n=5/445) (p<.001) Incidence rate was 3.6 per 100 p/y in E1 and 0.5 episodes per 100 p/y in E2. Median time from KTx to PTLD diagnosis was 27 months (IQR: 12.4-63.7). Ten developed early disease at 4.2 months (IQR: 3-6) and 29 late, at 31 months (IQR: 16-67) (p=.0008). On histological examination, plasmocytic hyperplasia was observed in 20, hyperplasia follicular: two, infectious mononucleosis: three, polymorphic: five, monomorphic lesions: 8, Hodgkin’s lymphoma: one. Main affected organs were tonsils:24, other nodes:7, small bowel:4, brain:2, liver:1; pancreas:1. Pat EBV negative pre KTx, had a mean 10.6 times higher risk (CI:95%: 5-21 p=.001) of developing PTLD, compared to those EBV positive. The 1-, 3-, and 5-yr pat survival with and without PTLD was 94.8%, 92% and 86,4%; and 99%, 97.6% and 96.3%, respectively (p=.001). The 1-, 3-, and 5-yr graft survival rate was 92.3%, 89,6%, and 87% and 93,4%, 86% and 81,5%, respectively (p=.35). Twelve pat EBV negative received rituximab (375mg/m2) in the peri KTx (Table2). None increased EBV loads or developed PTLD.
Conclusions: In this cohort, the incidence of PTLD decreased. EBV negative patients had on average a 10.6-fold increased risk of PTLD compared to those EBV positive. Patient survival was worse in those with PTLD. Administration of peri KTx Rituximab in EBV negative recipients was associated with the absence of PTLD during follow-up period.
[1] Lymphoproliferative disease
[2] Epstein-Barr Virus
[3] Rituximab
