Renal insufficiency causes rapid growth of pediatric kidney graft in a pediatric-to-adult kidney transplantation
Tianhui Pan1, Junbo Li1, Daqiang Zhao1, Gang Chen1.
1Institute of Organ Transplantation, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People's Republic of China
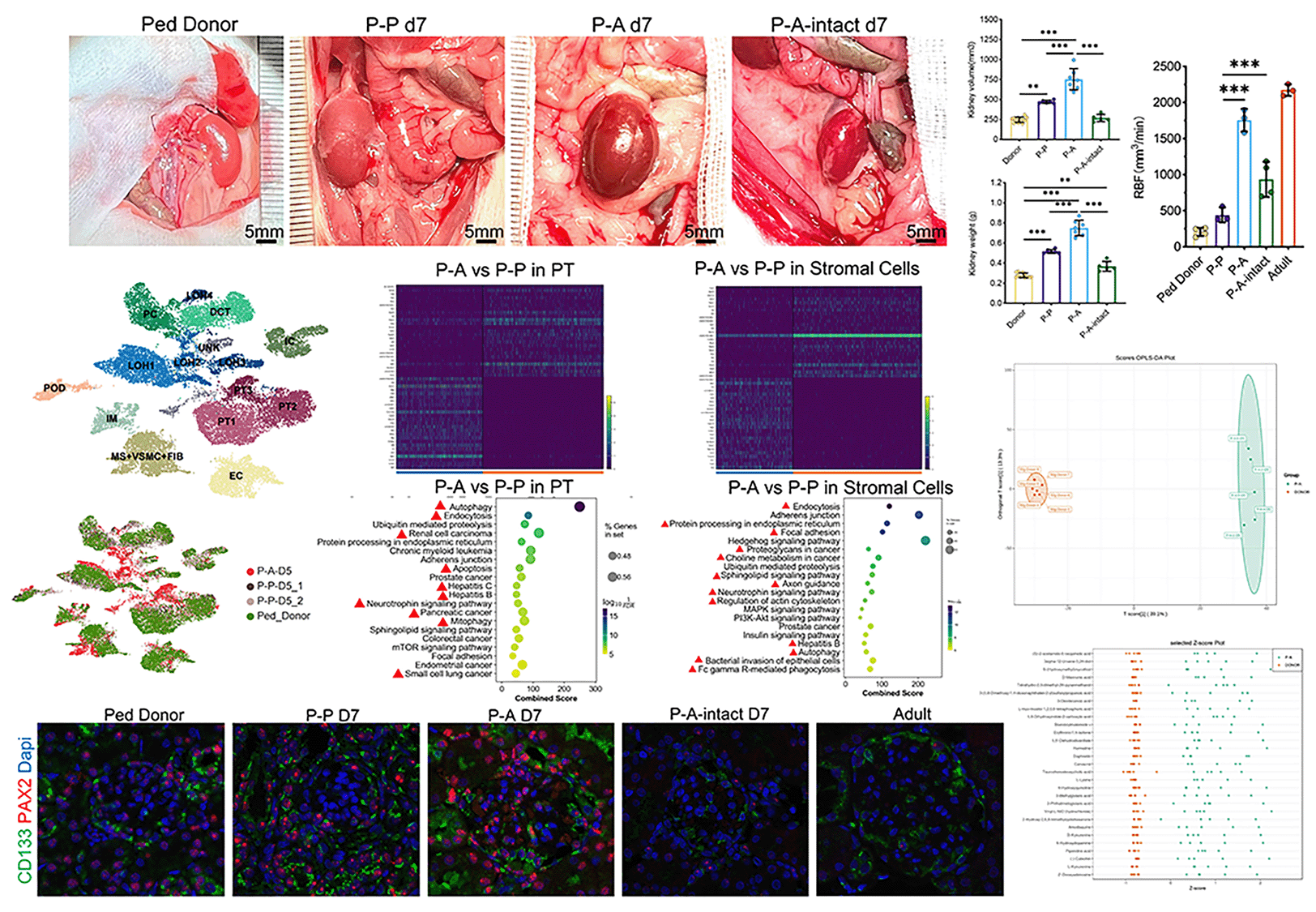
Pediatric kidney grafts exhibited rapid adaptive growth in the Pediatric-to-Adult transplantation scenario, which is different from the situation of weight-matched kidney transplantation. Animal models are valuable tools for disclosing the underlying mechanisms and impacts on long-term graft survival. After successfully constructing the rat model of Pediatric-to-Adult(P-A) kidney transplantation, we found that pediatric kidney exhibited the highest growth rate during the first week post transplantation. Then the pediatric kidneys were transplanted to different recipients including weight-matched pediatric rats(P-P), adult rats(P-A) and adult rats with both kidneys intact(P-A-intact). Recipients were sacrificed on day 7 after transplantation. Ultrasonography, biochemical test of serum and urine, blood pressure measurement, graft histological analysis by light and electron microscopies were performed before and after transplantation. Single-nucleus RNA sequence of whole kidney tissue and correspond metabolomics of serum were conducted on day 5 after transplantation. (Due to some bugs, unable to upload the figure) When the pediatric kidney was transplanted to adult recipient with bilateral nephrectomy(P-A), graft histology revealed enlargement of the glomeruli, thickening of GBM, as well as obvious hypertrophy and proliferation in tubular epithelial cells. SnRNA shown that dramatic changes happened in proximal tubule and stromal cells. DEGs between P-A and P-P group indicated that the PTECs in P-A graft were still in severe damage and underwent repairing and remodeling while the PTECs in P-P graft had recovered to normal. Accordingly, metabolomics indicated that Uremic Toxins especially those in tryptophan metabolism. At the same time, there appears more CD133+PAX2+ kidney progenitor cells in stromal cells. Though the renal blood flow (RBF) of graft in P-A-intact was twice of that in P-P kidney, the changes mentioned above were not observed in P-A-intact group. Besides, the normal growth of pediatric kidney was completely suppressed in the P-A-intact group. Which means the pediatric kidney will not grow if there have been enough nephrons. The P-to-A model reported here suffices to simulate the clinical P-to-A kidney transplant scenario. Our study indicates that insufficient kidney function rather than the hyper-perfusion of kidney plays a key role in driving the rapid growth of pediatric kidney graft. Kidney progenitor cells may play an important role in improving kidney function during the process of PTECs` regeneration under severe damage. Also, the reason why P-A kidney transplantation seemed to be underwent more severe damage is still wondering.
[1] Kidney Transplantation
[2] Kidney Growth
[3] Pediatric Donor
