Liver surface can be a promising transplant site for pancreatic islets using a gelatin hydrogel nonwoven fabric combined with ADSCs
Yukiko Endo Kumata1, Akiko Inagaki2, Yasuhiro Nakamura3, Takehiro Imura2, Shoki Suzuki1, Takumi Katano2, Ryusuke Saito1, Kazuaki Tokodai1, Takashi Kamei1, Michiaki Unno1, Kimiko Watanabe2, Yasuhiko Tabata4, Masafumi Goto1,2.
1Surgery, Tohoku University Graduate School of Medicine, Sendai, Japan; 2Transplantation and Regenerative Medicine, Tohoku University Graduate School of Medicine, Sendai, Japan; 3Pathology, Graduate School of Medicine, Tohoku Medical and Pharmaceutical University, Sendai, Japan; 4Plastic and Reconstructive Surgery, Graduate School of Medicine, Kyoto University, Kyoto, Japan
Introduction: Islet transplantation (Tx) is a promising treatment for type 1 diabetes patients. We previously demonstrated that islet Tx with syngeneic keratinocyte sheets onto the liver surface (LS), which can overcome the challenges of intraportal (IPO) injection, achieved glycemic control in diabetic rats, but issues including lot-to-lot variation persisted. Hence, this study explored a gelatin hydrogel nonwoven fabric (GHNF) as an alternative to keratinocyte sheets. Adipose tissue-derived stem cells (ADSCs) have also been shown to promote islet engraftment, yet their stickiness poses risks in IPO Tx. LS islet Tx with ADSCs could be a safe strategy for improving islet Tx outcomes. We aimed to establish a novel approach by transplanting islets onto the LS using a GHNF combined with ADSCs.
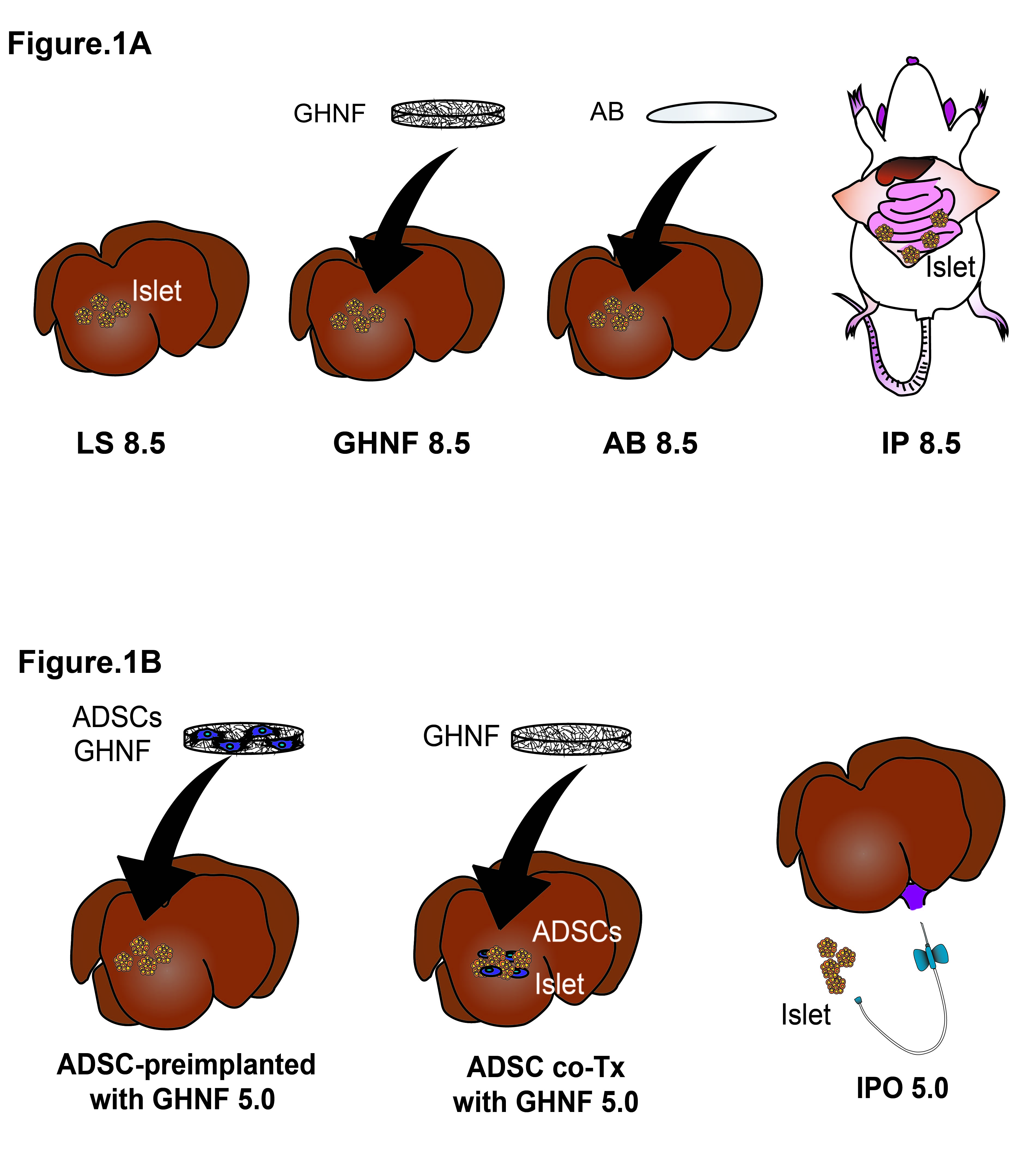
Method: In the experimental groups, 8.5 islet equivalents (IEQs)/g of syngeneic islets were transplanted onto the LS (Fig 1A). The transplanted islets were uncovered (LS 8.5), covered with the GHNF (GHNF 8.5) or covered with an adhesion barrier (AB 8.5). Intraperitoneal Tx (IP 8.5), in which islets were placed into the abdominal cavity, excluding the LS, served as a control group. To examine the additional effects of ADSCs, 5.0 IEQs/g of islets were transplanted onto the LS covered by ADSC-preimplanted GHNF (ADSC-preimplanted GHNF 5.0), or co-transplanted with ADSCs onto the LS covered by GHNF (ADSC co-Tx with GHNF 5.0, Fig 1B). These groups were also compared with the GHNF 5.0 group; the most optimized procedure was compared to the IPO 5.0 group. Graft function was evaluated by non-fasting blood glucose (BG) levels, intravenous glucose tolerance test, and cure rate. Extracellular matrix (ECM) and neovascularization were evaluated by immunohistochemistry. To elucidate the mechanisms of the effects of ADSCs, cytokines and growth factors in the culture supernatant were analyzed using an in vitro model.
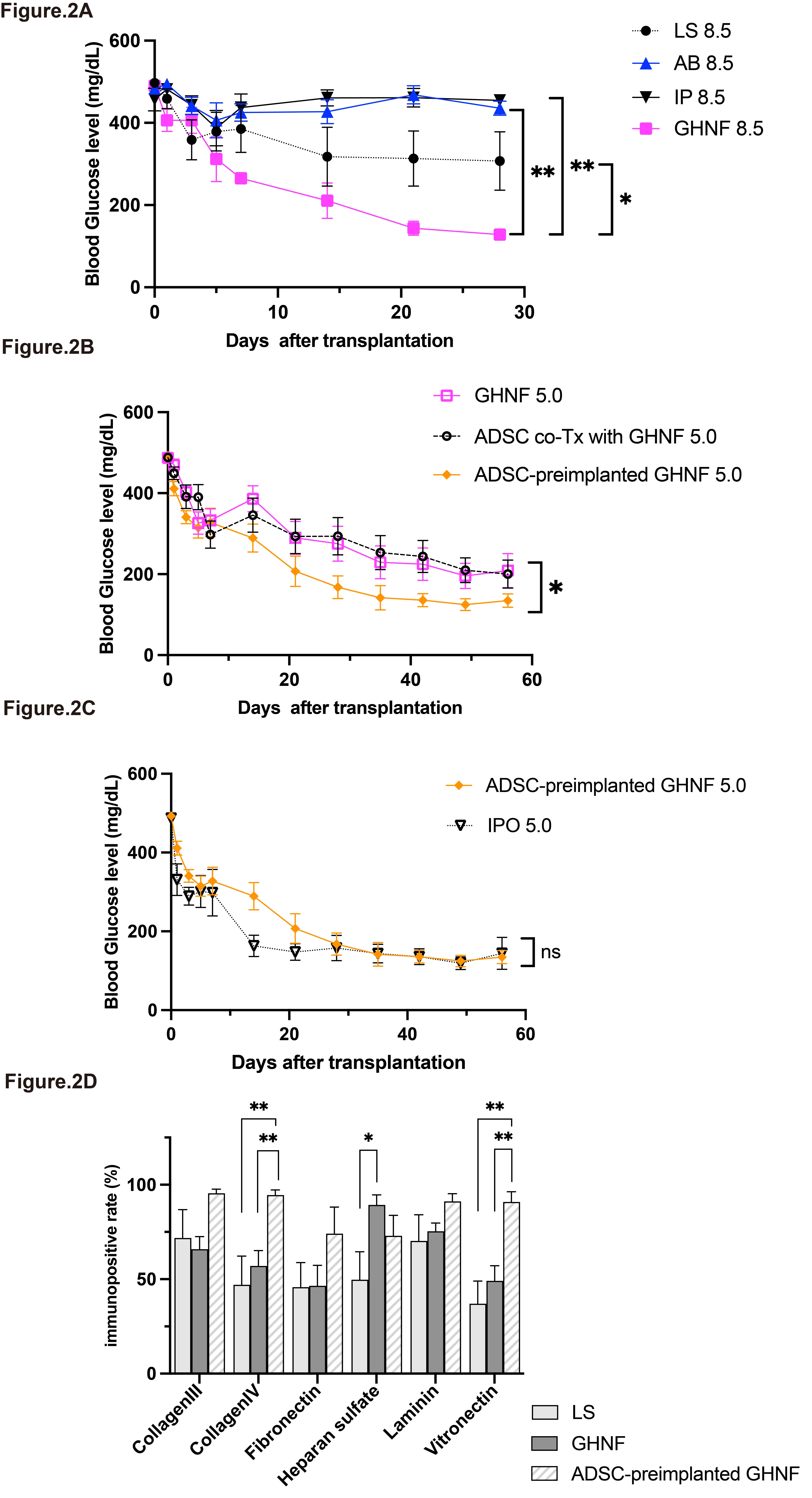
Results: The GHNF 8.5 group showed significantly lower BG levels (p<0.01, Fig 2A) and the highest cure rate (100%), whereas no cures was observed in the AB 8.5 or IP 8.5 groups (p<0.01). As for the additional effects of ADSCs, BG levels were significantly lower in the ADSC-preimplanted GHNF 5.0 group (p<0.05, Fig 2B). The cure rate was 92.3%, while that in the ADSC co-Tx with GHNF 5.0 and GHNF 5.0 groups was approximately 50% (p<0.05). The transplant outcome of the ADSC-preimplanted GHNF 5.0 group was comparable to that of the IPO 5.0 group (p=0.173, Fig 2C). In the ADSC- preimplanted GHNF group, several ECMs were enhanced at the LS (Fig 2D), whereas angiogenesis was not promoted in comparison to other groups. In the in vitro assay, we identified six molecules, including leptin, as crucial candidates for enhancing ECM compensation.
Conclusions: We established a novel method for LS islet Tx by combining the GHNF with ADSCs. The results were comparable to those of IPO Tx. These beneficial effects may be mainly due to the ECM compensation around the islets.
This study was performed according to a patent application agreement with KYOTO MEDICAL PLANNING Co., Ltd.
[1] islets
[2] gelatin hydrogel nonwoven fabric
[3] liver surface
[4] engraftment
[5] transplantation
[6] extracellular matrix
[7] neovascularization
[8] adipose tissue-derived stem cells
