
Vancomycin resistant enterococcus infection in multivisceral transplant and small bowel transplant recipients: A 10-year single center experience
Raul Rodriguez1, Lubna Osman-Kardash2, Jennifer Garcia1, Gennaro Selvaggi1, Jacques Simkins1, Lilian Abbo1, Vighnesh Vetrivel Venkatasamy1, Yoichiro Natori1, Rodrigo Vianna1, Shweta Anjan1.
1Miami Transplant Institute, Miami, FL, United States; 2Infectious Diseases, Mount Sinai Medical Center, Miami, FL, United States
Introduction: Solid organ transplant (SOT) recipients are at an increased risk for colonization and infection with Vancomycin resistant Enterococcus (VRE). Data on incidence and risk factors of VRE colonization and subsequent infection in Multi-visceral (MV), including isolated small bowel (SB), transplant recipients is limited.
Methods: This is a retrospective cohort study conducted at a large MV/SB transplant center in the US between March 1, 2013, and May 1, 2023. Immunosuppression regimen included anti-thymocyte globulin, steroids, and rituximab for induction, and tacrolimus, mycophenolate mofetil, and steroids as maintenance therapy. Institution preoperative antibiotic protocol includes vancomycin, cefepime, metronidazole and fluconazole. VRE surveillance with a rectal PCR was conducted prior to transplantation. VRE infection was defined as isolation of VRE from a sterile site. Incidence, risk factors, and outcomes of VRE infection including graft rejection and 90-day mortality were assessed.
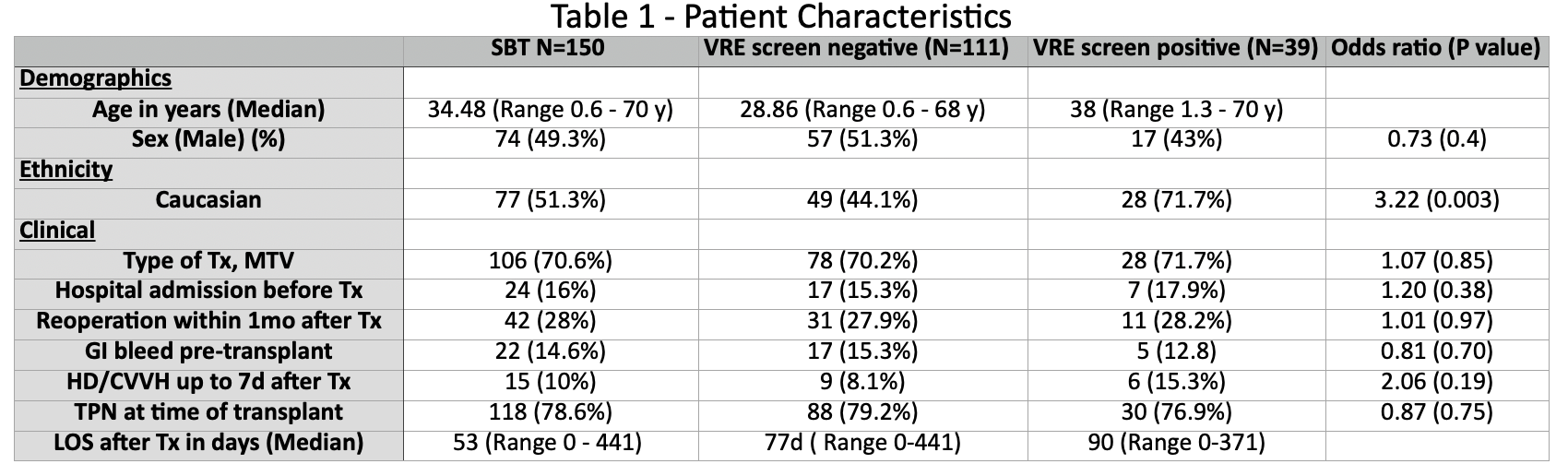
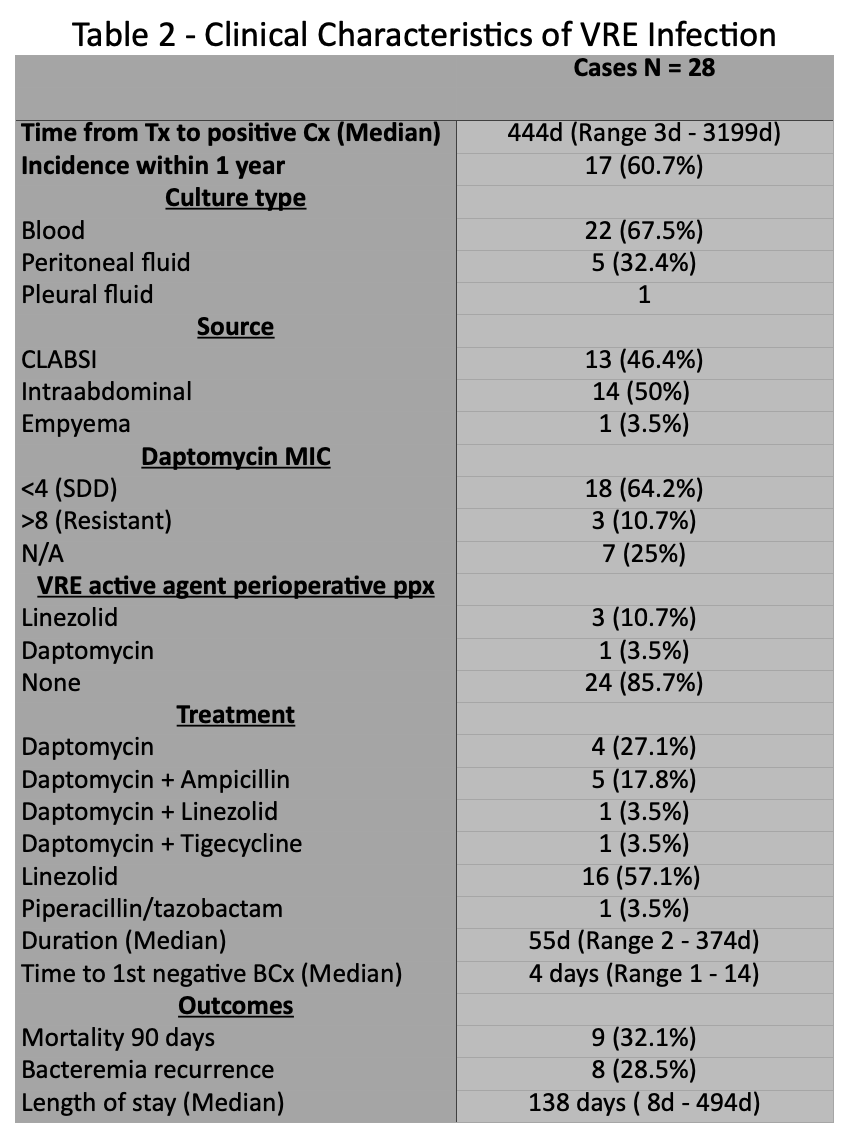
Results: We identified 150 consecutive patients (table 1); 74 (49.3%) were male, 45 (30%) Hispanic, with a median age of 34 (IQR 0.6 - 70). Thirty-nine (26%) had a positive VRE screen at any point during the study; Fifteen (10%) had a positive VRE screen prior to transplantation. Twenty-eight (18.6%) developed VRE infection (bacteremia or intra-abdominal infection) after transplant, with 16 (60%) occurring in the first year. Twenty-one (75%) bloodstream infections, 6 (21%) intra-abdominal and 1 (3.5%) pleural infection. Median duration of bacteremia was 4 days (range, 1-14). Bacteremia recurrence occurred in 8 (28.5%) patients. Positive VRE surveillance pre-transplant (OR 3.41 [95% CI: 0.94 to 12.26]; p 0.06) was a risk factor for VRE infection in the first year after transplant. Median length of hospital stay due to VRE infection was 138 days (range, 8-417d). At median follow-up of 1168 days (IQR 365 - 4307), two (7.1%) patients developed biopsy-proven rejection within 90 days after VRE infection and 90-day mortality in patients with VRE infection was 32.1% (9/28).
Conclusions: We report one of the largest studies on VRE infection in MV/SB transplant recipients. VRE surveillance and colonization can be used as a predictor for subsequent infection. Personalized perioperative antimicrobial prophylaxis should be employed in those known to be colonized prior to transplant.
[1] Multi visceral transplant.
[2] Small bowel transplant
[3] VRE
[4] Enterococcus
[5] Infection
[6] Surveillance
[7] Perioperative antibiotics
