Targeting the activity of beta cells by membrane surface redox regulation for islet transplantation
Jianmin Wu1, Changrong Shi2, Juan Chen1, Pengfei Rong1, Zijian Zhou2, Xiaoyuan Chen3, Wei Wang1, Xiaoqian Ma1.
1Cell Transplantation and Gene Therapy Institute, The Third Xiangya Hospital, Central South University, changsha, People's Republic of China, Changsha, Paraguay; 2State Key Laboratory of Vaccines for Infectious Diseases, Xiang An Biomedicine Laboratory & Center f, Xiamen University, Xiamen, China, Xiamen, People's Republic of China; 3Clinical Imaging Research Centre, Centre for Translational Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore, Singapore, Singapore
Introduction: Islet transplantation offers hope for type 1 diabetes treatment. While hypoxia-induced damage is one of the most important issues contribute to the graft loss in the early stage of islet transplantation. Given the weaker expression of the antioxidant system in beta cells compared to other cells, they are more susceptible to hypoxia-induced damage. Therefore, enhancing the resistance of beta cells to hypoxia damage has become a top priority. In this study, we designed fusogenic liposomes, termed Fulips, to regulate the survival and function of islet cells. The Fulips, equipped with 2,2,6,6-tetramethylpiperidine (TEMP) groups, decorated the membrane surface of islet cells after fusion, shielding them from oxidation-induced loss of activity by acting as ROS 'decoys' in the extracellular milieu. Additionally, ROS scavenging induced changes in molecular magnetism, quantitatively measurable through MRI relaxation time alterations.
Method: Islet cells activity was evaluated by flow cytometry, redox status by DCFH-DA fluorescence, and insulin levels post-glucose stimulation by ELISA. Neonatal porcine islets were extracted and subsequently transplanted under the renal capsule of NCG mice, serving as our standard transplantation model. Islet cells viability in grafts was assessed via TUNEL experiments on fixed pathology slices, while ROS content was evaluated using DHE pathological staining. All phantoms and animal MRI studies were performed on a Bruke 7.0 T small animal MRI scanner.
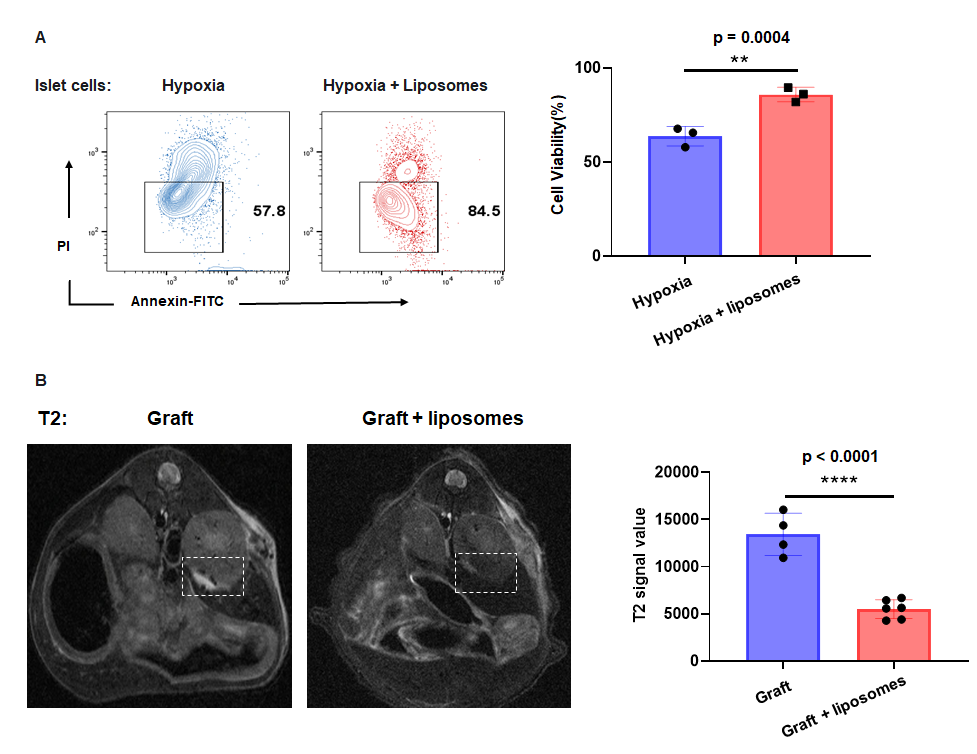
Results: In vitro, Fulips labeled cyanine 5.5 uniformly bound to islet cells membranes as observed by confocal microscopy. Under hypoxic conditions, the viability of islet cells was significant higher when co-cultured with Fulips than cultured alone (89.50±2.23% VS 67.60 ± 2.79%). Flow cytometry results showed a reduction in intracellular ROS levels of hypoxia islets cells after liposome treatment (79.20 ± 2.78% VS 55.70±2.47%). Hypoxia reduced insulin secretion by beta cells, while Fulips treatment reversed this trend. The islet cells co-cultured with Fulips were transplanted to the renal capsule of NCG mice, 3 days post-transplantation, magnetic resonance imaging indicated a reduction in T2 signal value (13419 ± 1120 VS 5499 ± 408) and shortened T2 relaxation time (119.0 ± 19.91 VS 43.80 ± 3.09) in the grafts which suggested reduced ROS levels and increased islet survival. TUNEL staining confirmed the results, that there was a reduction of apoptotic cells in liposomes co-cultured grafts compared to the control ones. DHE staining further demonstrated decreased ROS levels in liposome-treated grafts.
Conclusion: This work underscored the role of the redox status in bridging the activity of islet cells in situ and the treatment response for type 1 diabetes therapy. Ultimately, targeting the redox metabolism of translated cells by chemical approaches could realize modulation and molecular imaging of treatment response in transplantation therapy.
National Natural Science Foundation of China (Grant No: 82272102).
[1] Allograft Survival
[2] Islet transplantation
[3] Reactive Oxygen Species
[4] Liposome
