Episomal DNA vector-induced EPO-overexpressing kidney organoids exhibit physiological effects after implantation
Zhaoyu Du1, Amanda Bas-Cristóbal Menéndez1,2, Manuela Urban3, Anna Hartley3, Danielle Ratsma4, Marijke Koedman4, Thierry van den Bosch5, Marian Clahsen-van Groningen5, Joost Gribnau6, Jaap Mulder2, Marlies Reinders1, Carla Baan1,4, Bram van den Eerden4, Richard Harbottle3, Martin Hoogduijn1,4.
1Transplant institute, Erasmus MC, Rotterdam, Netherlands; 2Pediatrics, Sophia Children's Hospital, Erasmus MC, Rotterdam, Netherlands; 3DNA Vectors Lab, German Cancer Research Center (DKFZ), Heidelberg, Germany; 4Internal Medicine, Erasmus MC, Rotterdam, Netherlands; 5Pathology, Erasmus MC, Rotterdam, Netherlands; 6Developmental Biology, Erasmus MC, Rotterdam, Netherlands
The kidney's endocrine function is essential for maintaining body homeostasis. Erythropoietin (EPO) is one of the key endocrine factors produced by the kidney, and kidney disease patients frequently experience impaired EPO production, leading to anemia.Current treatments do not offer a long-term solution, and imply cardiovascular risk such as thrombosis. In the present study we explored the potential of human induced pluripotent stem cell (iPSC)-derived kidney organoids to restore EPO production in a mouse model.
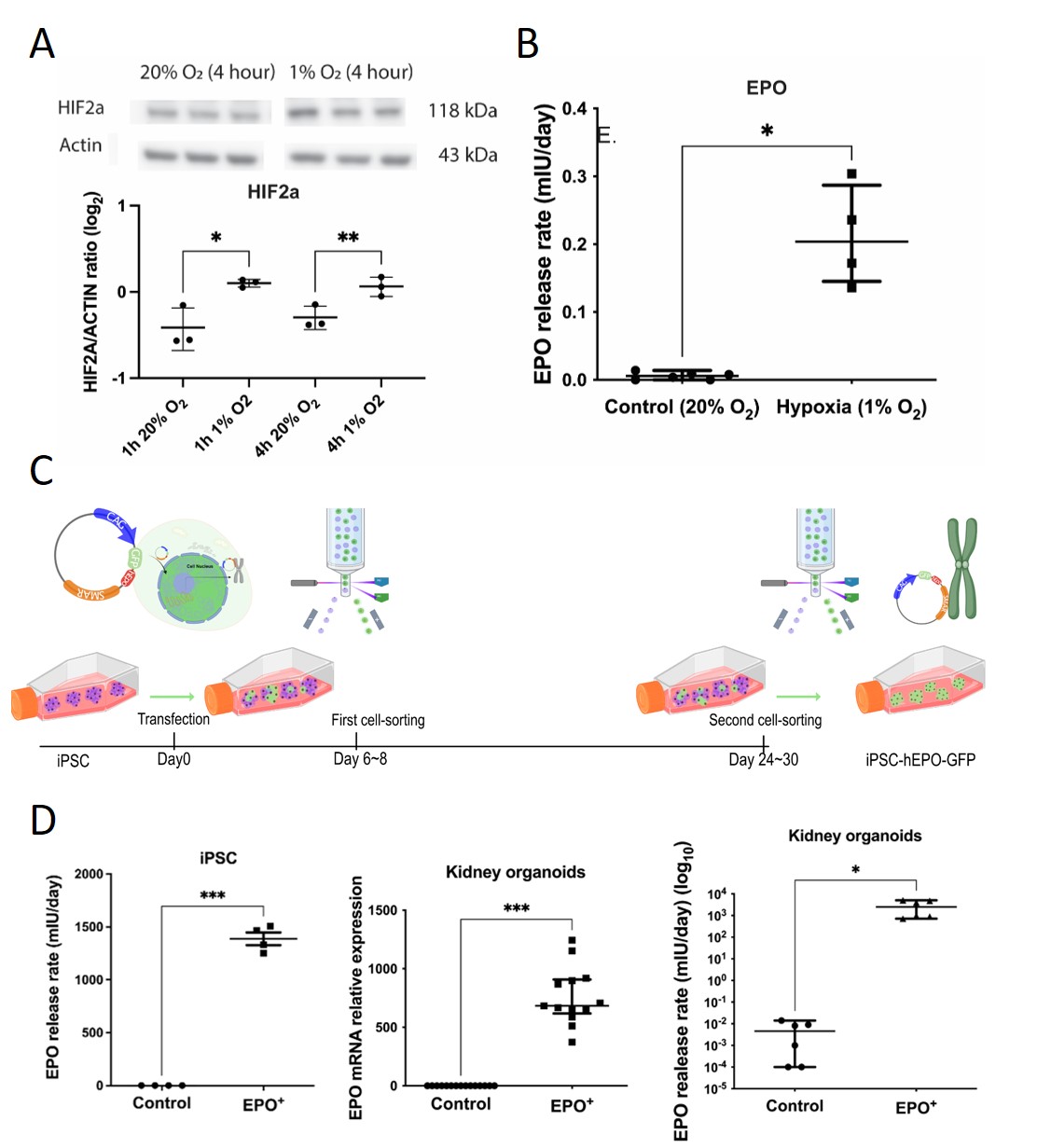
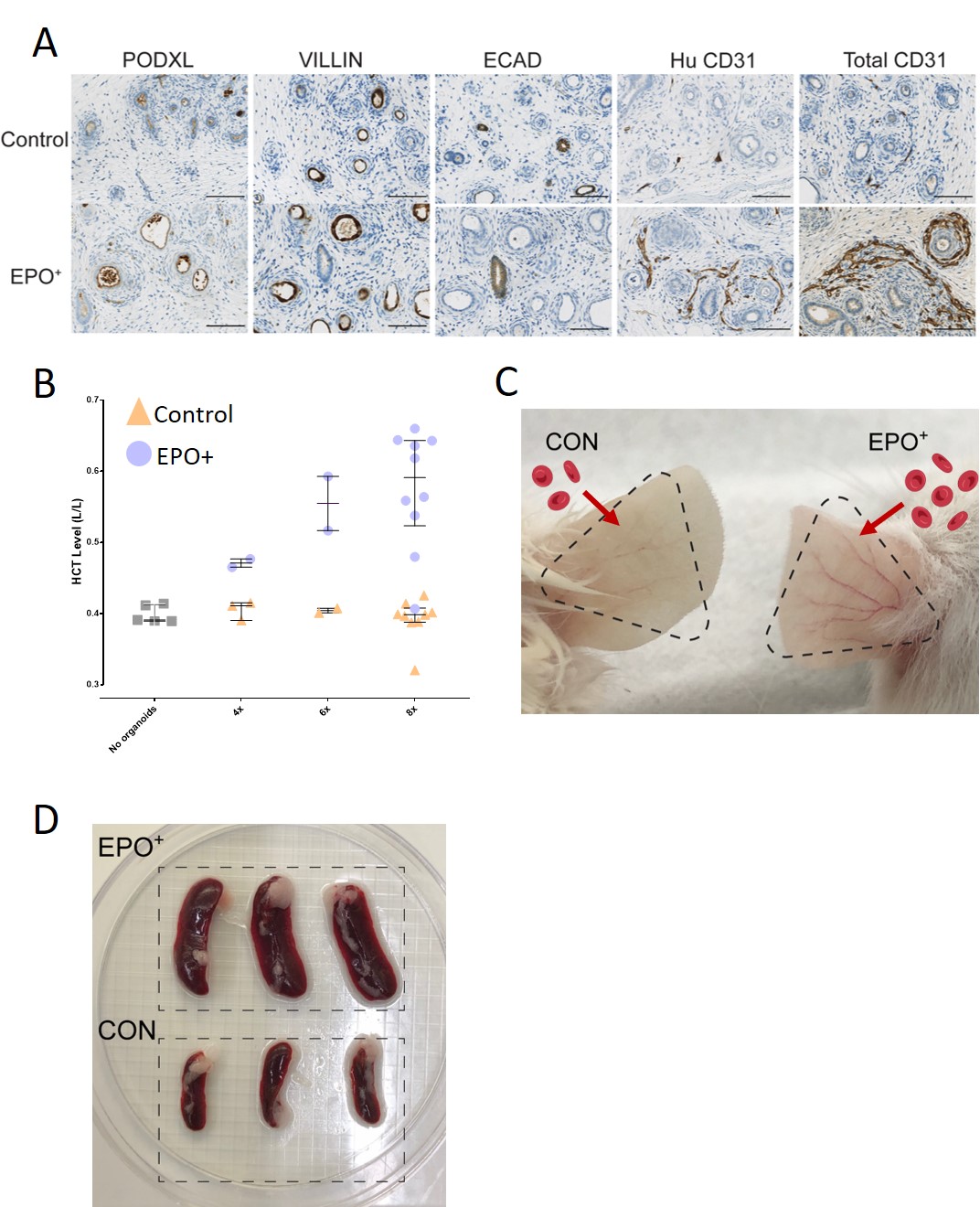
Kidney organoids produced low amounts of EPO under hypoxic conditions (Fig1 A,B) and therefore we designed a non-integrating scaffold/matrix attachment region (S/MAR) DNA vector containing the EPO gene (alongside GFP) to transfect iPSC and generate EPO overexpressing (EPO+) kidney organoids (Fig1 C). EPO S/MAR DNA vectors remained robustly expressed during iPSC expansion and kidney organoid differentiation (Fig1 D). Quantitative analysis of immunohistochemical staining revealed increased staining of PODXL for podocytes and PECAM for endothelial cells, alongside a reduction in E-cadherin staining for distal tubular cells in EPO+ organoids (Fig2 A). To assess the physiological effects of EPO+ organoids, 2-8 organoids were implanted subcutaneously in immunodeficient mice. One month post-implantation, EPO+ organoids displayed continuously elevated EPO mRNA levels and significantly increased endothelial cells compared to control organoids. Crucially, hematocrit levels were notably elevated in mice implanted with EPO+ organoids in an organoid number-dependent manner (Fig2 B,C). Moreover, a dramatic increase of the spleen size was noticeable in mice that received EPO+ organoids vs. control (Fig2 D). Additionally, EPO+ organoids increased osteoblast-stimulating fibroblast growth factor 23 mRNA expression in the bone marrow and affected trabecular bone composition.
In conclusion, S/MAR DNA vectors enable stable transgene expression throughout kidney organoid differentiation. The observed physiological effects following the implantation of EPO+ organoids underscore the potential of gene-edited kidney organoids for endocrine restorative therapy.
[1] Kidney
[2] EPO
[3] Organoids
[4] iPSCs
[5] Regenerative Medicine
[6] Implantation
