Development of an automated multi-sensor normothermic kidney perfusion device for ex-vivo preservation and assessment
Franka Messner1, Marlene Pühringer1, Michael Mengel3, Andrew Capulli2, Scott Coull2, Piper Curtis2, Joshua Filgate2, Steven Henning2, Molly Holmes2, Bradley Hovan2, Stuart Jacobson2, Sara Mills2, Tim Moreau2, Andrew Nelson2, Connor Stadnicki2, Alec Woods2, Stefan Schneeberger1.
1OrganLife® Laboratory, Medical University of Innsbruck, Innsbruck, Austria; 2DEKA Research & Development Corp, Manchester, NH, United States; 3Department of Laboratory Medicine and Pathology, University of Alberta, Alberta, AB, Canada
Background: Static cold storage and hypothermic machine perfusion remain the current standard preservation strategy for kidney grafts. Normothermic machine perfusion (NMP) has the potential to improve organ preservation, extend preservation times and allow for assessment of organ quality. We herein present a next-generation automated kidney perfusion device that allows for stable normothermic machine perfusion while providing a platform for extensive functional assessment of organ quality.
Methods:The prototype NMP device consists of a perfusion module, disposable set, and transport module. The device includes a low-hemolysis pump, an array of perfusate sensors, auto-regulated pressure and temperature, and oxygen delivery and glucose infusion that automatically adapts to the metabolic needs of the graft in real-time. These features result in a device that provides unprecedented control, monitoring, and assessment of donor kidneys. Perfusate and urine samples can be easily collected for further assessment. A large touchscreen serves as the user interface and displays all perfusion parameters in real-time or as a history, with cloud connectivity. Urine flow and color are measured, and the device can be configured to either recirculate or divert urine. A live video recording preserves the sterile environment while offering macroscopic assessment and retrospective analysis of the kidney appearance during NMP.
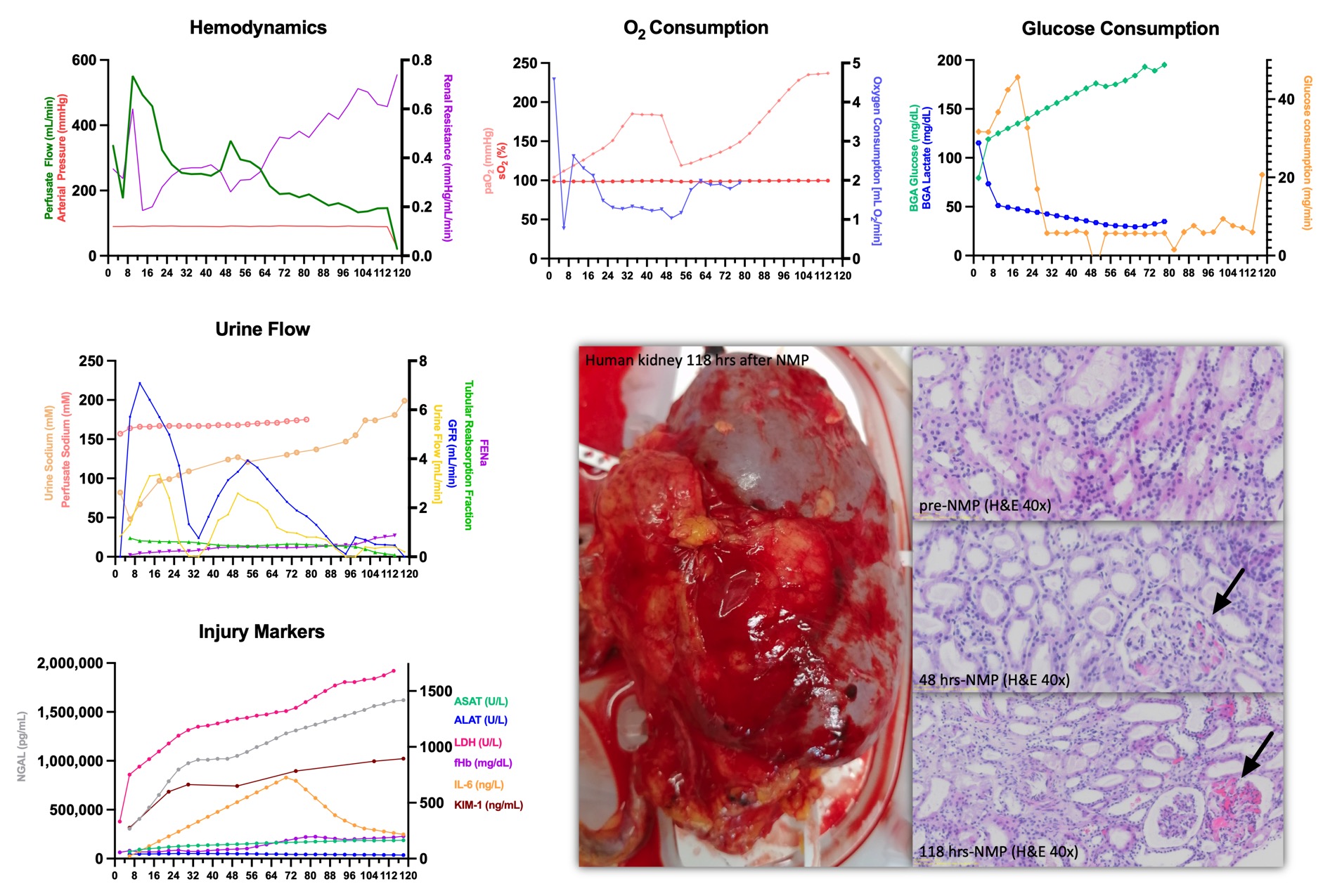
Results: 162 porcine and 69 discarded human kidney grafts were perfused resulting in over 8,100 hours of kidney perfusion. Preservation times for human kidney grafts were extended to 48 hours in 11 kidneys and further to a maximum of 118 hours in one human kidney. In this kidney, perfusion flow was stable (median 230 ml/min) at 90 mmHg, but eventually declining after day 4 to 150 ml/min. Stable oxygen and glucose consumption (median 1.62 mL/min, 5.99 mg/min), lactate clearance, pH regulation and uninterrupted urine flow (0.82 mL/min, GFR 1.78 mL/min) were found. Over the course of perfusion, a moderate rise in injury markers was noted. Macroscopic assessment as well as histology show preserved tissue architecture (Banff score after 118 hours of NMP: i0, t0, g0, ptc0, v0, ti0, i-IFTA0, t-IFTA0, ci0, ct0, cg0, mm1, cv0, ah2) with few glomerular microthrombi after multi-day perfusion.
Conclusion: The unique design and features of this novel NMP device enable next-generation automated kidney perfusions with minimal need for post-reperfusion interventions and provide a platform for comprehensive ex-vivo graft assessment.
[1] normothermic machine perfusion
[2] kidney preservation
[3] ex-vivo organ preservation
[4] organ assessment
[5] prolonged perfusion
[6] automated multi-sensor device
[7] kidney perfusion
[8] graft assessment
