
Dr Michael Collins MBChB FRACP PhD is a transplant nephrologist at the Royal Adelaide Hospital and a Clinical Senior Lecturer at the University of Adelaide. After completing a PhD in transplant immunology at the University of Adelaide, he has worked as a nephrologist and clinical researcher in Auckland, New Zealand, and Adelaide, South Australia. He proposed and is the co-lead investigator of the BEST-Fluids trial, a multi-centre, pragmatic trial of IV fluid therapy in kidney transplant recipients. This work was supported by the Jacquot Research Establishment Fellowship, in addition to funding from the Medical Research Future Fund (Australia), the New Zealand Health Research Council and the BEAT-CKD group of investigators. He is the Deputy Chair of the Australasian Kidney Trials Network Scientific Committee, and an active clinical trialist. His ongoing research focuses on designing and conducting clinical trials of interventions that will improve outcomes for kidney transplant recipients.
A pilot randomised controlled trial of advanced recovery room care post living donor kidney transplantation
Karthik Venkataraman1,2, Guy Ludbrook2,3, Toby Coates1,2, Michael Collins1,2.
1Central and Northern Adelaide Renal and Transplantation Service, Royal Adelaide Hospital, Adelaide, Australia; 2Faculty of Health and Medical Sciences, University of Adelaide, Adelaide, Australia; 3Department of Anaesthesia, Royal Adelaide Hospital, Adelaide, Australia
Background: Significant variation exists between hospitals in the management of kidney transplant recipients in the immediate post-operative period. Post-operative care settings described in the literature include intensive care units, high dependency units and renal wards. No comparative data exists to inform practices. Advanced Recovery Room Care (ARRC) is a model of post-operative care that provides a high dependency unit level of care, under the supervision of an anaesthetist and trained critical care nurses. ARRC was designed, among other things, to detect and address post operative hypotension. Post operative hypotension has been linked to post-operative adverse cardiovascular events and may adversely affect graft function. ARRC has been shown, in non-transplant settings, to increased days at home within 30 days post operatively and reduce medical emergency responses post operatively, chiefly by reducing hypotension. We sought to investigate the safety, feasibility and efficacy of ARRC in the post-operative management of kidney transplantation.
Methodology: We conducted a single-centre pilot open-label randomised controlled trial (ACTRN12622001093774), randomising live donor kidney transplant recipients 1:1 to either post operative care in the ARRC or to standard of care management on the renal ward. The intervention involved closer haemodynamic monitoring, more frequent medical officer review and the ability to assess and address post operative hypotension with fluids and vasopressors. The primary outcomes were 1) safety (adverse events in the first 28 days post transplantation), 2) recruitment feasibility and 3) episodes of hypotension between groups, defined as a systolic blood pressure of less than 100 mmHg or a mean arterial pressure under 70mmHg.
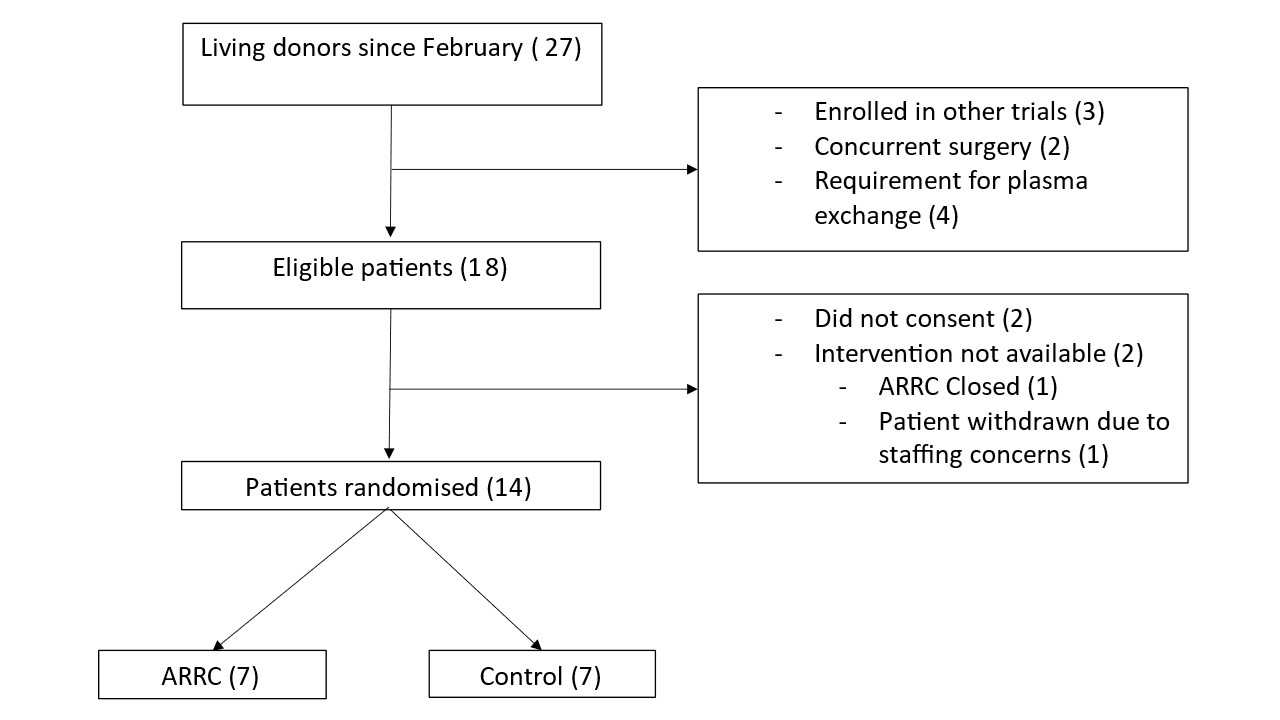
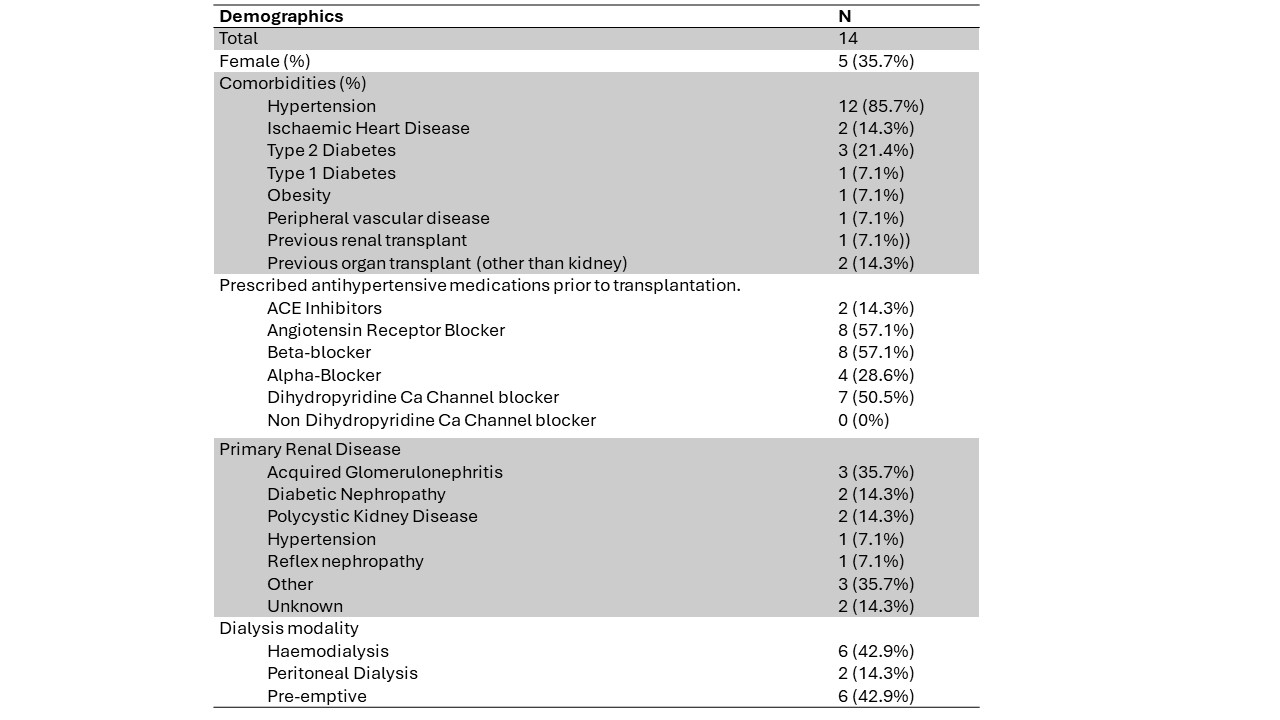
Results: An interim analysis was performed after 11 months of recruitment (Jan 2024), after approximately 75% of the recruitment target was met. 27 living donor transplants have been performed during the study to date; 18 recipients were eligible for enrolment into the trial. A total of 14 participants underwent randomisation (Figure 1). There were no major adverse events in either arm. 4 out of 7 participants (57%) were hypotensive post operatively in the control arm compared to 1 out of 7 participants (14%) in the intervention. The trial is ongoing.
Conclusion: In the interim analysis of this pilot trial, use of advanced recovery room care was safe, and recruitment into a larger clinical trial appears feasible. Advanced recovery room care may reduce the incidence of post-transplant hypotension.
Dr Venkataraman is supported by a grant from Kidney, Transplant & Diabetes Research Australia.
[1] Kidney transplantation
[2] Post operative management
[3] Advanced Recovery Room Care
