
Impact of VRE-active perioperative prophylaxis in liver transplant patients colonized by Vancomycin-Resistant Enterococci
Giulia Burastero1, Emmanuel Q Wey2,3, Veronica Guidetti4, Samuele Cantergiani1, Davide Lo Porto5, Valentina Serra6, Giacomo Assirati6, Fabrizio Di Benedetto6, Giovanni Guaraldi1, Marianna Meschiari1, Adriana Cervo1, Giovanni Dolci1,8, Alessandra Mularoni5, Massimo Girardis7, Cristina Mussini1, Erica Franceschini1.
1Infectious Diseases Unit, Azienda Ospedaliero-Universitaria of Modena, Modena, Italy; 2Department of Infection, Royal Free London NHS Trust London, London, United Kingdom; 3Centre for Clinical Microbiology, Division of Infection & Immunity, UCL, London, United Kingdom; 4FIM Department, Universitiy of Modena and Reggio Emilia , Modena, Italy; 5Unit of Infectious Diseases, IRCCS-ISMETT Istituto Mediterraneo per i Trapianti e Terapie ad Alta Specializzazione, Palermo, Italy; 6Hepato-Pancreato-Biliary Surgery and Liver Transplantation Unit, University of Modena and Reggio Emilia, Modena, Italy; 7Anesthesia and Intensive Care Medicine, University of Modena and Reggio Emilia, Modena, Italy; 8Azienda Unità Sanitaria Locale, Modena e Reggio-Emilia, Modena, Italy
Background: Data regarding the effectiveness of vancomycin resistant enterococci (VRE) active prophylaxis in pre-liver transplant (LT) VRE-colonized patients for preventing early post-LT VRE infections are scarce. The aim of this study is to assess the impact of VRE-active prophylaxis on the development of early VRE infections in pre-LT VRE-colonized patients.
Methods: retrospective, observational, multicenter cohort study involving AOU of Modena, IRCCS- ISMETT in Palermo, and Royal Free Hospital in London. All pre-LT VRE colonized patients transplanted between January 1, 2010 and July 31, 2023 were enrolled. The incidence of early-onset VRE infection (bloodstream infections, superficial and deep surgical site infections within 30 days post-LT) was evaluated comparing patients with VRE-active prophylaxis versus patients with VRE non-active prophylaxis t LT.
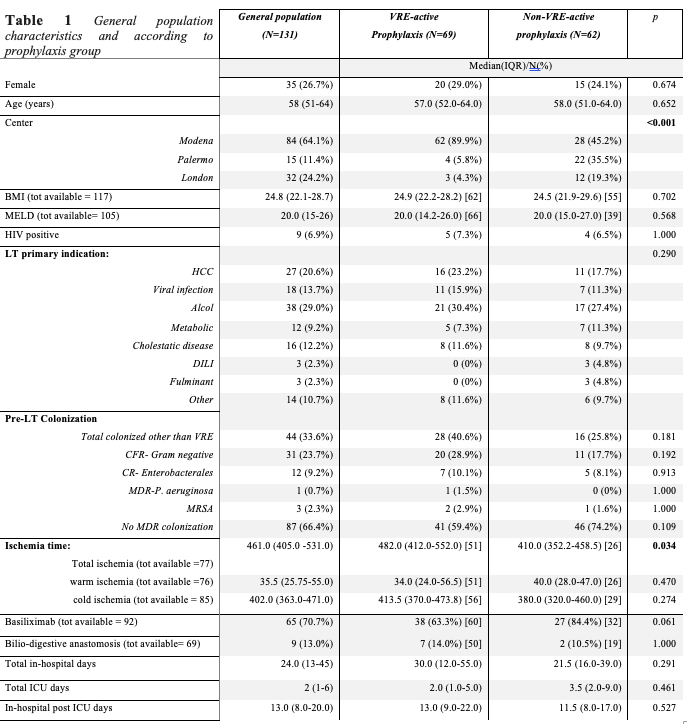
Results: One-hundred-thirty-one patients were enrolled, 62 (47.3%) and 69 (52.7%) patients received “non-active” and “active” VRE prophylaxis, respectively. Tigecycline was the most common drug prescribed in the “active” prophylaxis group (55/69 cases, 79.7%). Characteristics according to prophylaxis groups are shown in Table 1.
No significant difference in the number of patients who developed early-onset VRE infection between the VRE-active and the non-VRE-active prophylaxis group was observed [6 (8.7%) vs 8 (12.9%) cases, p=0.621, respectively]. Outcomes are depicted in Table 2.
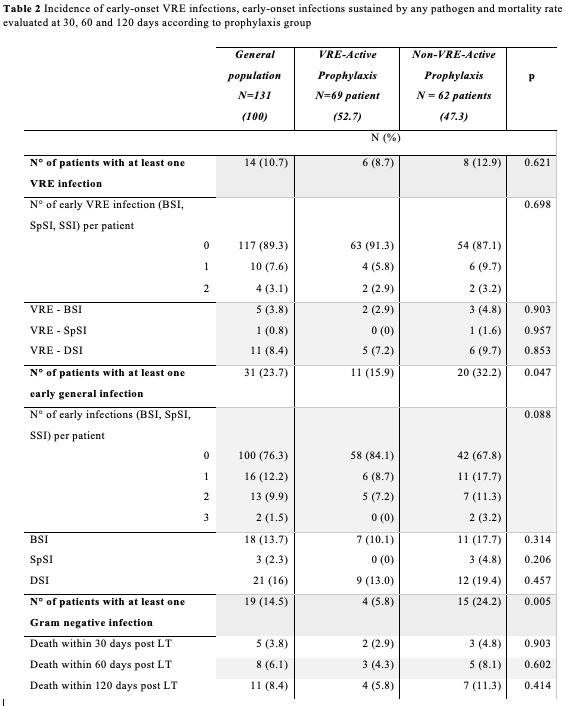
Total ICU-days (OR 1.04 CI 1.005-1.085, p=0.028) and bilio-digestive anastomosis (OR 6.122 CI 1.068-35.105, p=0.042) were associated with higher risk of developing early VRE infection at multivariate analysis. General early infections were significantly lower in the “active” VRE prophylaxis group compared to the control group [11 (15.9%) vs. 20 (32.2%), p=0.047]. Bilio-digestive anastomosis (OR 193.591 CI 1.364-27.478.046, p=0.037) and “active” prophylaxis with tigecycline (OR 0.012 CI 0.000-0.4482 p=0.019) was associated with a higher and lower risk of early general infection respectively in multivariate analysis.
Conclusion: our study shows that VRE- active prophylaxis in VRE colonized patients at LT is ineffective in preventing post-LT VRE infections and for this reason should not be recommend. Nevertheless, our results underlined a possible role of tigecycline as LT perioperative prophylaxis in a setting with high ESBL colonization to reduce early post-LT infections sustained by any pathogen. In order to confirm these findings, further investigations, possibly randomized and on large population, are needed.
[1] Surgical Site Infection
[2] Vancomycin Resistant Enterococci (VRE)
[3] Perioperative Prophylaxis
[4] OLT
[5] VRE target prophylaxis
