Survival benefit of living donor availability for pediatric liver transplantation in a large North American center: An intention-to-treat analysis
Zhihao Li1, Owen Jones1, Fernanda Takamatsu3, Jennifer Stunguris3, Gonzalo Sapisochin1, Mark Cattral1,2, Anand Ghanekar1,2, Asad Siddiqui4, Vicky L. Ng3, Blayne A. Sayed1,2.
1HBP & Multi-Organ Transplant Program, University Health Network, Toronto, ON, Canada; 2Division of General and Thoracic Surgery, Hospital for Sick Children, Toronto, ON, Canada; 3Division of Gastroenterology, Hepatology, and Nutrition, Hospital for Sick Children, Toronto, ON, Canada; 4Department of Anesthesia and Pain Medicine, Hospital for Sick Children, Toronto, ON, Canada
Background: Living-donor liver transplantation (LDLT) is increasingly adopted for pediatric LT. However, its specific benefits are underexplored. This study conducts an intention-to-treat analysis from listing time to assess LDLT's effectiveness in the pediatric population.
Methods: Pediatric LT candidates (<18years) listed between 2001-2023 at a single Canadian center were categorized as pLDLT (with an evaluated potential live donor) or pDDLT (without a live donor). Multiorgan transplant and retransplant candidates were excluded. Employing Cox proportional-hazard regression, we evaluated pLDLT’s survival impact through a covariate-adjusted analysis (age, sex, Pediatric End-Stage Liver Disease (PELD) score, etiology, body weight <5kg, listing period).
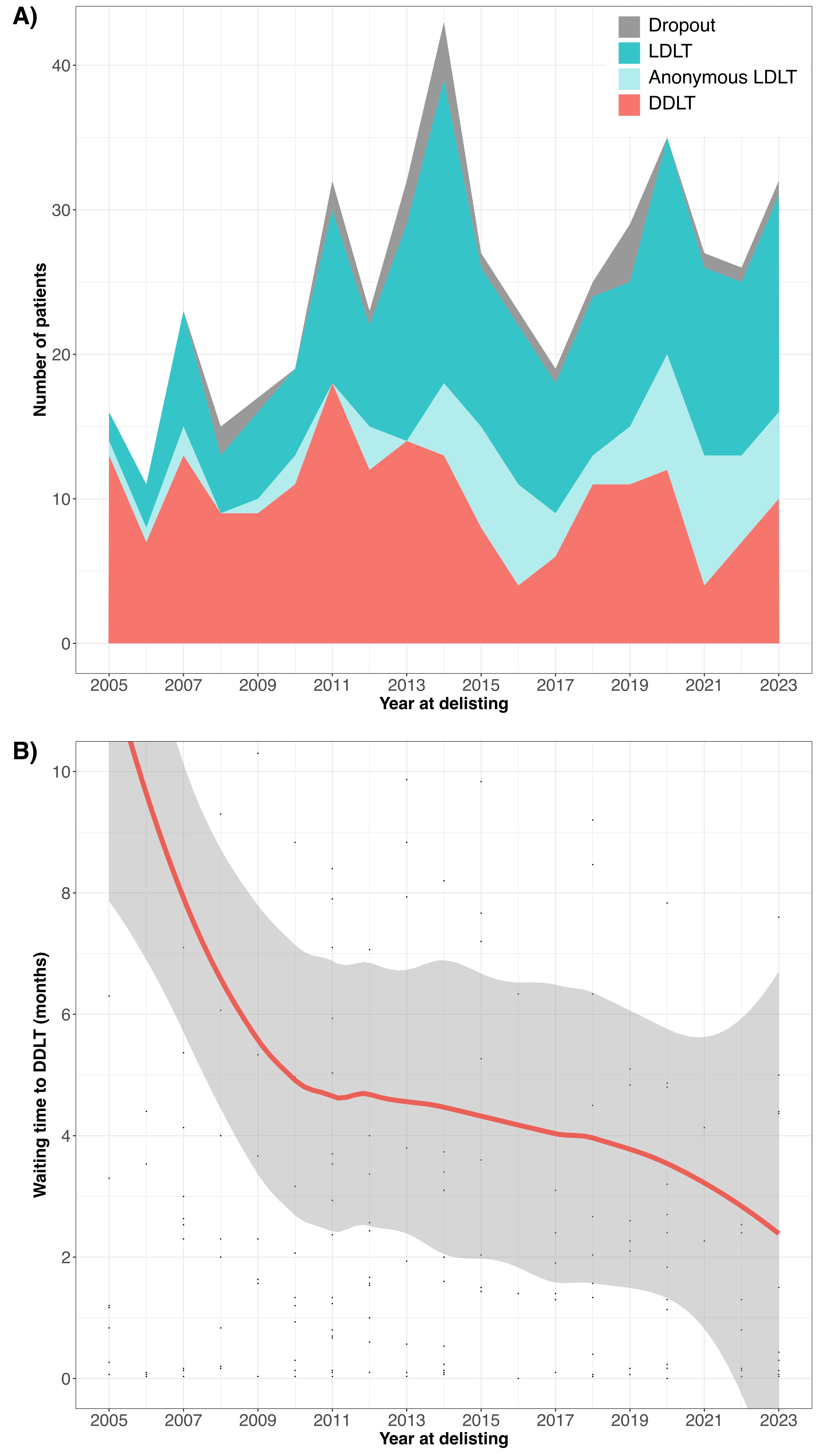
Results: Among 474 candidates, 219 (46%) had potential live donors. The pLDLT group was younger than the pDDLT group (median: 9 (IQR:5-41) vs. 28 (IQR:6-108) months, p<0.001). The most common etiology was biliary atresia (pLDLT:53%, pDDLT:28%), while acute liver failure was more prevalent in the pDDLT group (20% vs. 6%, p<0.001). In the pLDLT group, the waitlist dropout rate was 0.5% (n=1), compared to the pDDLT group with a significantly higher dropout rate of 9% (n=23). The median wait time was shorter for pLDLT than pDDLT (1.9 (IQR:1.2-3.9) vs. 2.6 (IQR:1.0-5.2) months, p=0.04). Superior survival rates in the pLDLT group were observed at 1-, 5-, and 10-years post-listing (98.6%, 96.6%, 96.6% vs. 87.6%, 84.4%, 83.1%, log-rank p<0.001) (Figure1A) with an 72% reduced mortality risk (aHR 0.28, 95% CI 0.12-0.64, p=0.003). The survival benefit persisted in the subgroup analysis for biliary atresia and acute liver failure (Figure1B&C). In the as-treated analysis, 67 pDDLT candidates benefited from anonymous living donation, resulting in 258 (57%) receiving LDLT and 192 (43%) DDLT. LDLT recipients demonstrated superior survival (log-rank p=0.003) (Figure1D) with a 65% mortality risk reduction (aHR 0.35, 95% CI 0.13-0.93, p=0.036). Although the number of patients listed annually increased over the study period, the waiting time for DDLT has shortened, potentially connected to an increase in live donors (Figure2A&B).
Conclusion: Having a potential live donor is associated with substantial survival benefit. Pediatric programs offering LDLT can expand the donor pool and decrease the waiting time for DDLT. Given its overall superior outcomes, LDLT may become the preferred standard for pediatric liver transplants.
Figure 1: Survival outcomes of intention-to-treat and as-treated analyses.
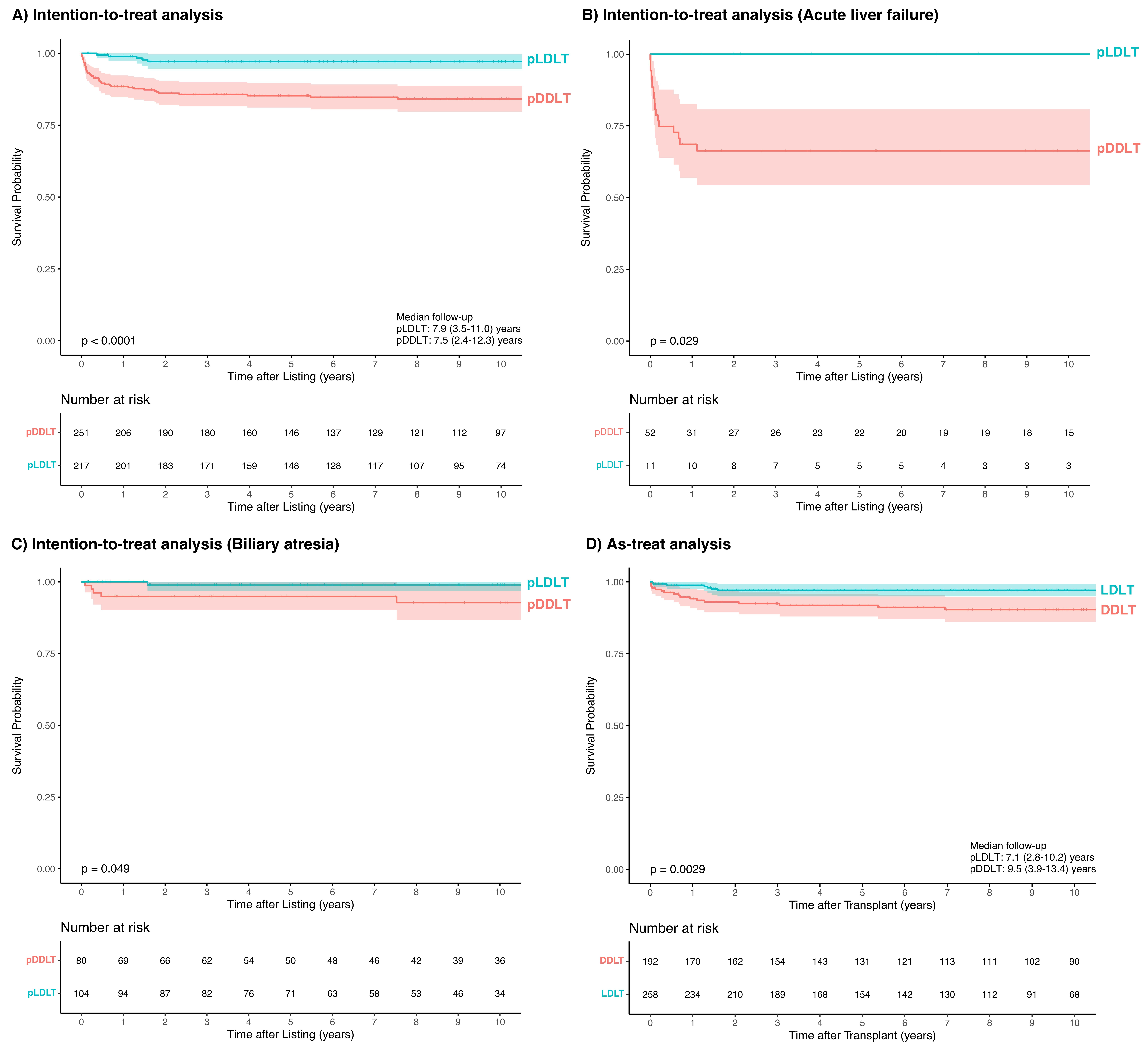
Figure 2: Temporal changes of waitlist outcomes and waiting time.