Outcomes of intestinal transplant in a single large adult centre
Bala Mahesh Polamreddy1, Irum Amin1, Terence Tan1, Charlotte Rutter1, Neil Russell1, Dunecan Massey1, Jeremy Woodward1, Lisa Sharkey1, Andrew Butler1.
1Transplant Surgery, Cambridge University Hospitals NHS Foundation Trust, Cambridge, United Kingdom
Introduction: There has been a significant improvement in intestinal transplantation (ITx) outcomes. This is due to a combination of advances and innovations in peri and post operative care and surgical techniques (including the management of immunosuppression and infections). Despite this, recipients still experience a burden of mortality and morbidity which is greater than for other solid organ transplants.
Methods: A retrospective analysis of a prospectively maintained database from Dec 2007 to Feb 2024 was undertaken. Data fields analysed included recipient age, indication for transplant, type of graft, mortality rates, explants, length of stay (LOS), episodes of rejection and patient survival.
Results: A total of 156 ITx were performed in 150 patients including 6 re-transplants. The median age at transplant was 45 years (16-65 years). 77% were male and 71%were female.
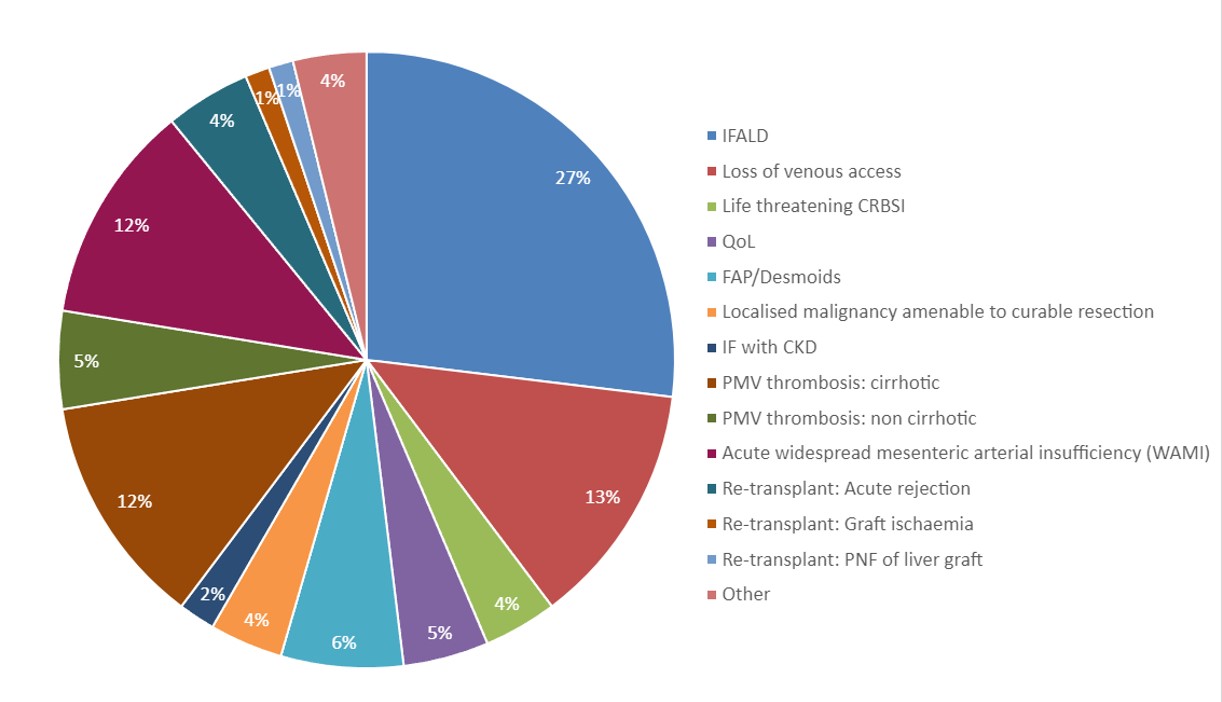
Indications for transplant are shown in Figure 1 (66 for intestinal failure and related complications, 35 for widespread mesenteric ischaemia, 16 for extensive abdominal evisceration, 9 other).
Fifty-five small bowel (SBT), 17 modified multivisceral (MMVT), 35 liver small bowel (LSB) and 49 multivisceral (MVT) were performed. SBT includes the pancreatico-duodenal complex to stabilise vascular structures and preserve the middle colic vessels to allow colon transplantation.
Immunosuppression involved alemtuzumab & methylprednisolone (MP) induction, maintenance of tacrolimus, prednisolone and MMF or azathioprine. Rejection was initially treated with pulsed MP, but severe, exfoliative rejection with varying combinations of ATG, plasma exchange and alemtuzumab. There have been 58 episodes of rejection, and 9 patients required a small bowel explant. One patient was treated with eculizumab for severe AMR of the liver in a LSB.
Nineteen (12%) recipients were treated for PTLD. The EBV status of the donor (D) and recipient (R) for these patients were 5 (D+, R-), 6 (D-, R+), 8 (D+, R+). Initial treatment for PTLD cases was with rituximab and 3 patients needed second line treatment with RCHOP chemotherapy and 2 patients were treated with cell-based therapies.
The rate of CMV viraemia was 20% (32/156). 9/32 were (D+/R-). A change in donor recipient matching strategy resulted in a drop in CMV viraemia rates.
The median LOS was 70 days (18 – 522 days). In 2023 the median LOS was 34 days as the majority were SBT. In the financial year 2022/2023 18 adult ITX were performed.
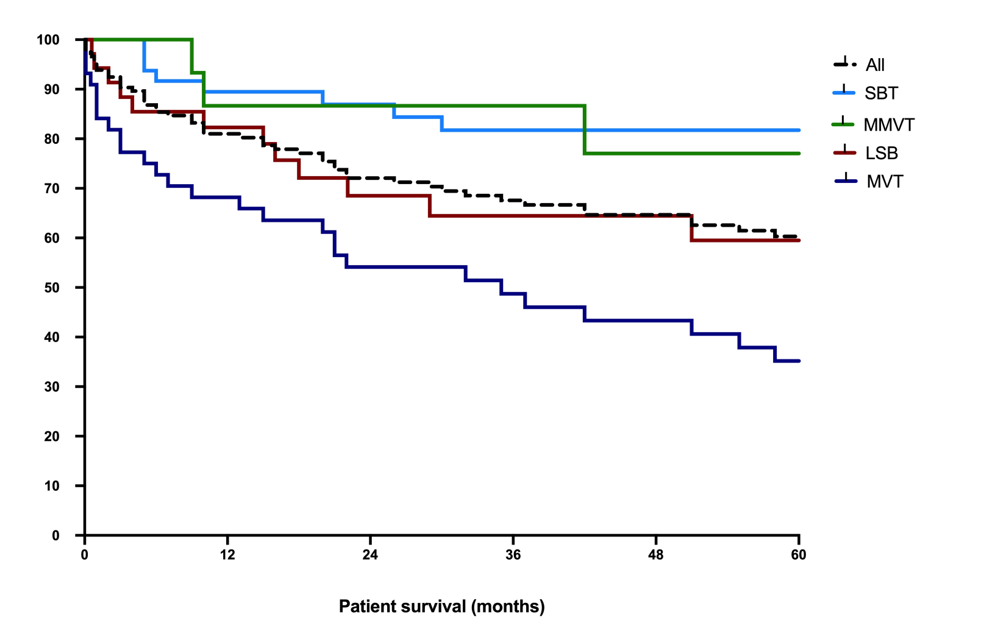
Currently there are 74 (47.4%) patients alive. Figure 2 shows the survival rates.
Conclusion: This is an adult only single centre study over a 16-year period describing the patient outcomes and complication rates.
[1] intestinal transplant
[2] intestinal failure
[3] Graft rejection
[4] immunosuppression
[5] Allograft survival
[6] multivisceral
[7] modified multivisceral
[8] bowel transplant
