Outcomes of redo-liver transplantation using hypothermic oxygenated machine perfusion: A multicentre cohort study (REDO-HOPE)
Damiano Patrono1, Rebecca Panconesi1, Silvia Zamboni4, Paolo Magistri4, Arianna Trizzino3, Riccardo De Carlis6, Stefania Camagni2, Duilio Pagano7, Alex Borin5, Amedeo Carraro5, Domenico Pinelli2, Salvatore Gruttadauria7, Luciano De Carlis6, Davide Ghinolfi3, Fabrizio Di Benedetto4, Renato Romagnoli1.
1General Surgery 2U - Liver Transplant Unit, AOU Città della Salute e della Scienza di Torino, Turin, Italy; 2Department of Organ Failure and Transplantation, ASST Papa Giovanni XXIII, Bergamo, Italy; 3Division of Hepatic Surgery and Liver Transplantation, University of Pisa Hospital, Pisa, Italy; 4Hepato-pancreato-biliary Surgery and Liver Transplantation Unit, University of Modena and Reggio Emilia, Modena, Italy; 5Department of General Surgery and Dentistry, Liver Transplant Unit, University Hospital of Verona, Verona, Italy; 6General Surgery and Abdominal Transplantation Unit, Niguarda-Cà Granda Hospital, Milan, Italy; 7Department for the Treatment and the Study of Abdominal Diseases and Abdominal Transplantation, IRCCS-ISMETT, UPMC Italy, Palermo, Italy
Background: Redo-liver transplantation (LT) is a challenging operation and is associated with an increased rate of postoperative complications and reduced graft and patient survival. Although optimal grafts are normally used for redo-LTs, these might not be readily available. By improving preservation and allowing prolonging preservation time, hypothermic oxygenated machine perfusion (HOPE) may improve outcomes of redo-LT.
Methods: REDO-HOPE is a multicentre cohort study aimed at investigating outcomes of redo-LT performed using grafts treated by HOPE. Adult (≥18-year-old) recipients of a second or third liver transplant performed between January 2016 and December 2023 will be included. Baseline recipient and donor characteristics, as well as procedural data, will be collected. Outcomes of HOPE redo-LT will be compared with benchmark values and with those of a matched group of redo-LT performed using static cold storage. Primary endpoint will be 12-month graft survival. Early redo-LT are defined as those performed within 60 days from the index LT. Extended criteria donors (ECD) are defined as those with one of the following: donation after circulatory death (DCD), age>65years, BMI≥40, steatosis≥30%, Na>165mmol/L, ICU stay≥7 days, or cold ischemia time > 10 hrs.
Results: So far, data on 23 redo-LTs (early, n=7; late, n=16) performed using HOPE at 7 Italian centres were collected. Median (IQR) HOPE time was 145 (101-248) minutes. There was a progressive increase of caseload during study period, with most cases performed in 2023 (Figure 1). Median MELD-Na at transplant was 15.4 (7.5-23); 15 (65.2%) were admitted in hospital, of whom 5 (21.7%) in ICU; 6 (26.1%) were on life support and 1 (4.5%) on dialysis (Table 1). An ECD graft was used in 15 (65.2%) cases. Notably, median donor age was 61.8 (34-74.2) years, and 6 (26.1%) livers were from DCD donors, all procured using normothermic regional perfusion before ex-situ HOPE. The rate of early allograft dysfunction, dialysis requirement and Clavien grade≥3b complications was 39%, 15% and 52%. Five patients died postoperatively. Outcomes were inferior in early redo-LTs, although differences were not statistically significant. 12-month patient and graft survival were 78.3% (63%-97%) and 73.9% (58%-94.2%). Graft survival was unaffected by donor ECD status.
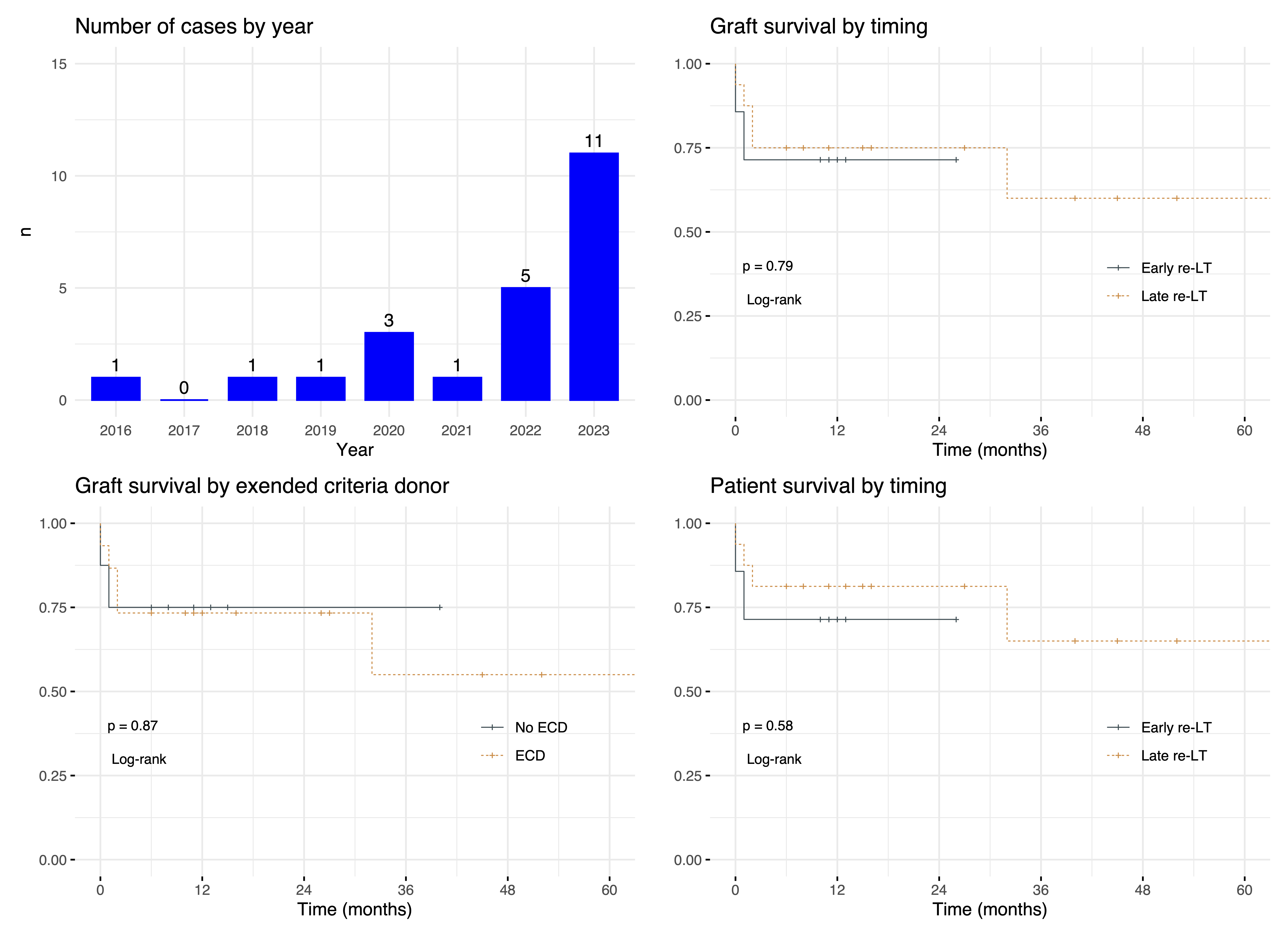
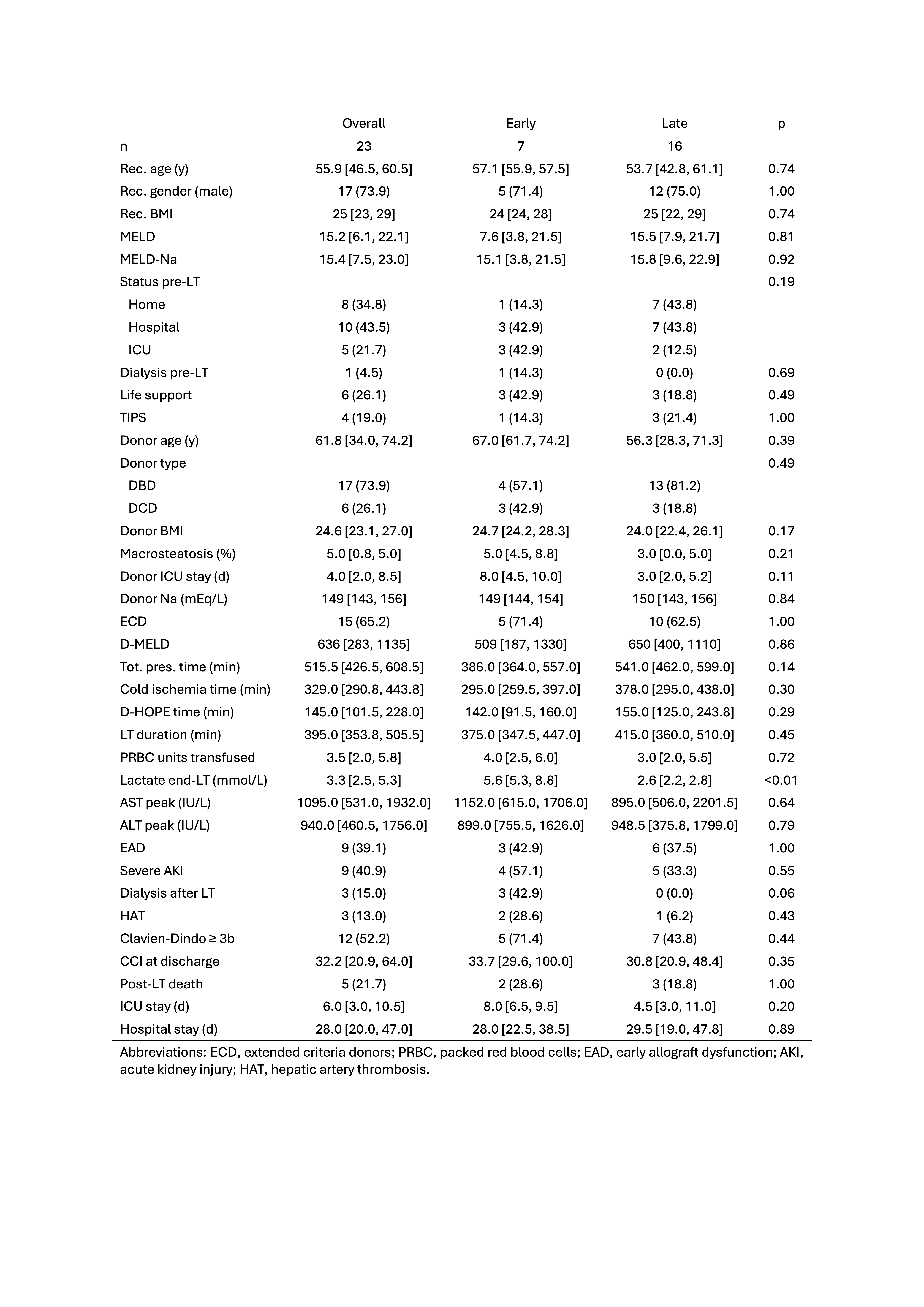
Conclusion: According to this preliminary analysis, HOPE may allow performing redo-LT with outcomes comparable to benchmark values despite the use of ECD grafts.
[1] Retransplantation
[2] Redo-liver transplantation
[3] Hypothermic oxygenated machine perfusion
