CD38 promotes regulatory T cells survival by activating the calcium/Ras/GGT1 pathway to reduce ROS and induce post-transplant immune tolerance
Jinren Zhou1, Jian Gu1, Yunjie Lu2, Qufei Qian1, Ling Lv3.
1Hepatobiliary Center, The First Affiliated Hospital of Nanjing Medical University , Nanjing, People's Republic of China; 2Soochow University, Suzhou, People's Republic of China; 3Affiliated Hospital of Xuzhou Medical University, Xuzhou, People's Republic of China
Introduction: Thousands of liver transplantations have been performed worldwide to treat end-stage liver diseases, yet only a few may induce transplant tolerance. Therefore, understanding the immune equilibrium in tolerant patients has become a prominent focus in transplantation research. Regulatory T cells (Tregs) constitute a subset of T-cells with immunomodulatory roles crucial for maintaining immune balance. Numerous studies have highlighted the pivotal role of Tregs in regulating liver immunity post-transplantation, either through exogenous injection or endogenous induction of Tregs. Tregs were also shown to reduce acute graft-versus-host disease (GVHD) when transferred after transplantation into mice, which was dependent on IL-2. GVHD is a major hurdle for successful stem cell transplantation in patients.
A significant challenge for Tregs in maintaining immune tolerance after liver transplantation lies in understanding how Tregs persistently control immune balance in vivo. Data from mice or human in vitro settings suggest that Tregs may not endure for extended periods and could lose their suppressive capacity to manage pathological immune responses. Recent research suggests that Tregs require increased ATP to sustain their suppressive function, leading to higher susceptibility to ROS-induced injury. ROS produced by neutrophils can cause tissue damage after transplantation and impair Treg function. This factor is critical and cannot be overlooked in influencing Treg's lifespan.
We aim to investigate the functions and mechanisms associated with specific Treg subsets in the transplantation immune tolerance environment and to determine the effect of CD38 on Tregs.
Method: Mass Cytometry, Cell metabolism measurement, lentiviral transduction et al.
Results: CD38hi Tregs demonstrated higher activation levels and proliferative capacity, more robust suppression of CD8+ T cells, lower ROS levels, and better long-term persistence. In a humanized mouse GVHD model, compared to CD38low Tregs, the infusion of CD38hi Tregs was more effective in delaying the process of transplantation immune rejection. These findings are consistent with the role of CD38 in other lymphocytes. Based on whole protein analysis and metabolomics, CD38hi Tregs were enriched in the Ras/NF-κB signaling pathway in KEGG enrichment analysis, and the dominant metabolic pathway was glutathione metabolism. GGT1 ranked first among the proteins upregulated in CD38hi Tregs during glutathione metabolism. Additionally, NF-κB (as a TF) also helped to promote the transcription of GGT1. A reduction in GGT1 damages the proliferative capacity and cell viability of Tregs by attenuating GSH levels and increasing cellular iron death levels.
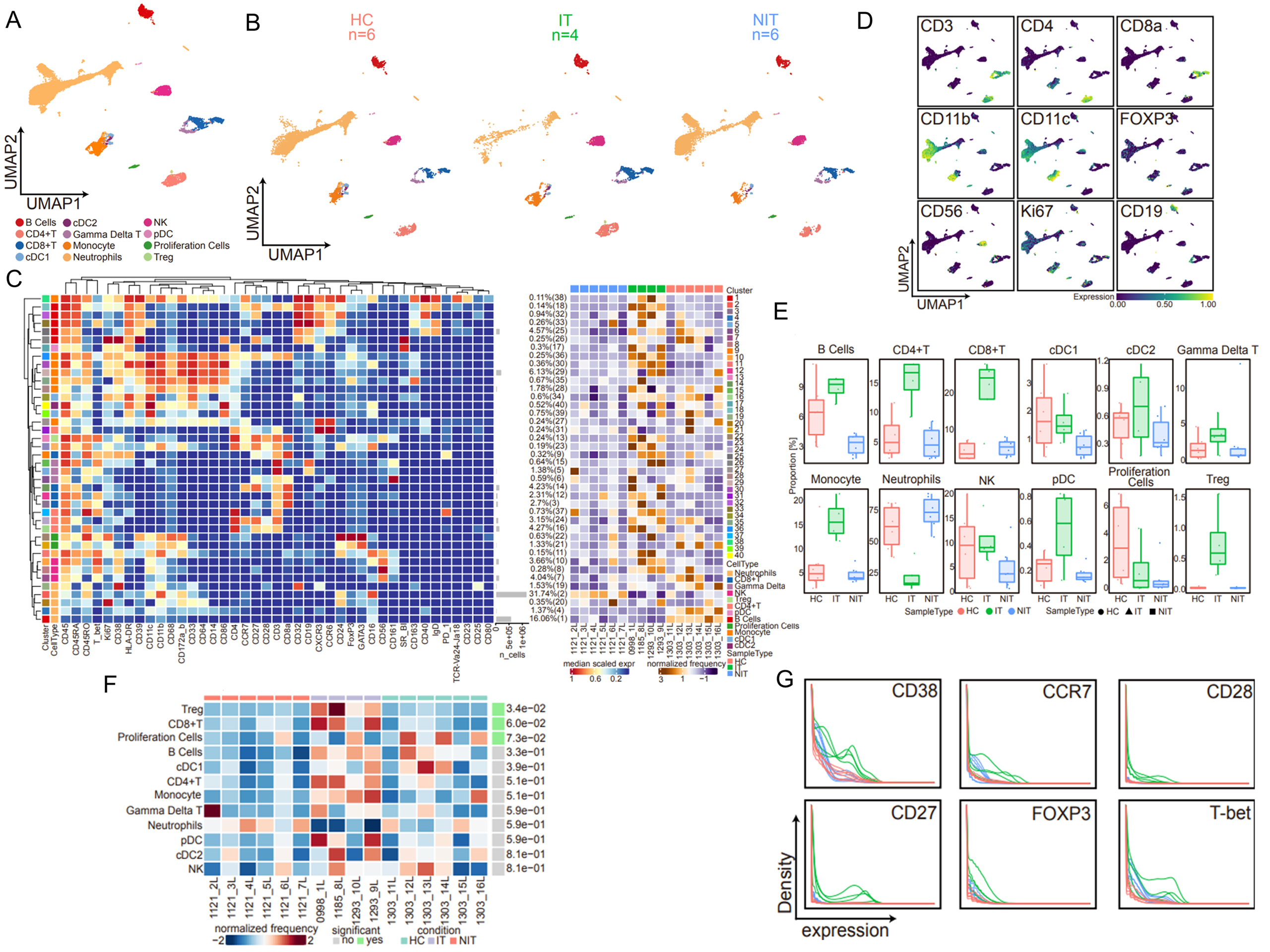
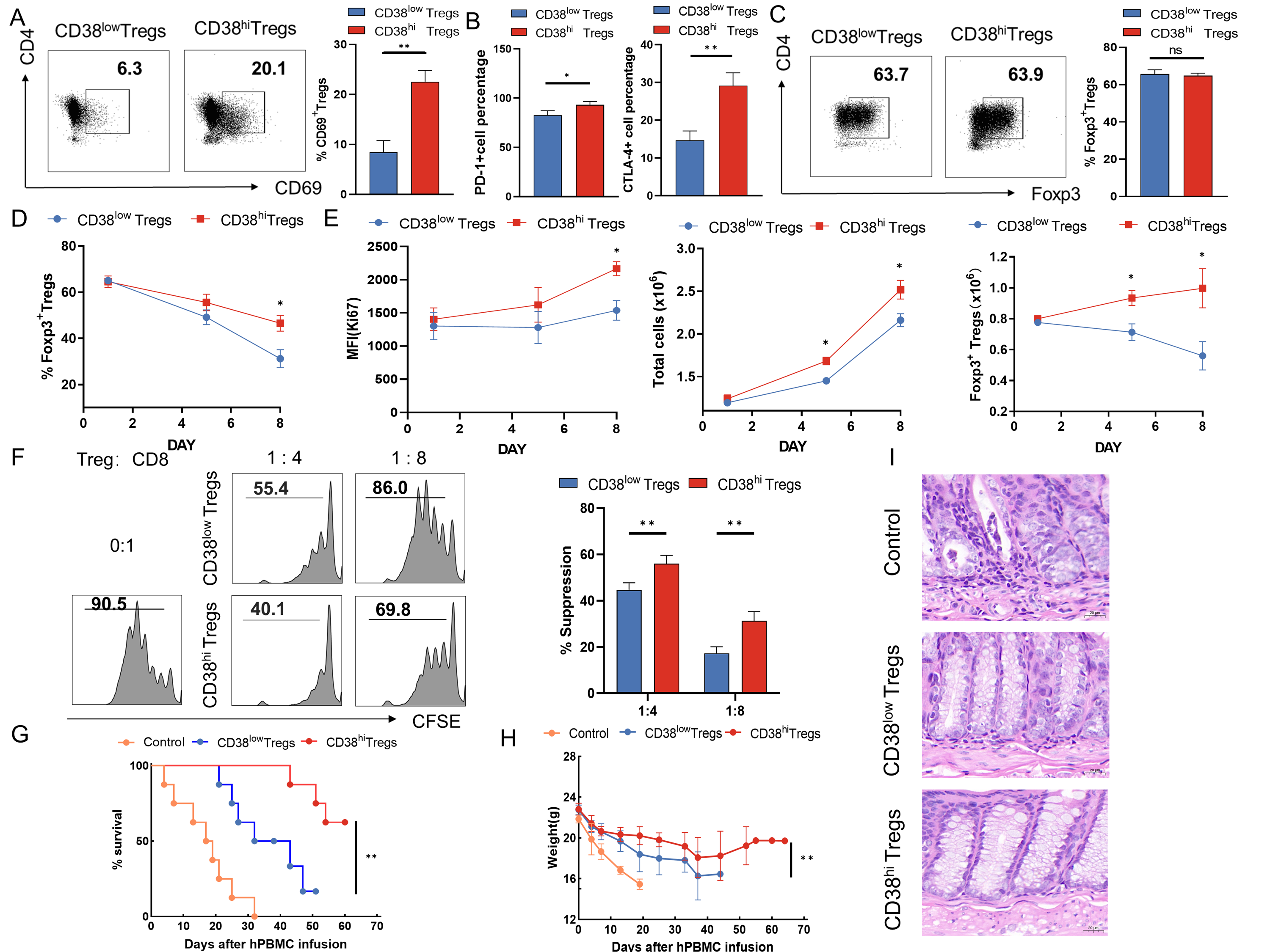
Conclusion: Our study contributes to a better understanding of the mechanisms involved in liver allograft immune regulation and provides a potentially useful biomarker and therapeutic for inducing tolerance in solid organ graft recipients.
This study was supported by grants from the National Natural Science Foundation of China (82171759, 82101873), Natural Science Foundation of Jiangsu (BK20201486), the Foundation of Jiangsu Collaborative Innovation Center of Biomedical Functional Materials, 2022 Jiangsu Graduate Research and Innovation Program (SJCX22_0668, KYCX22_1842, KYCX23_1930), 2023 Jiangsu Science and Technology Association youth Science and technology talent lifting project (JSTJ-2023-XH015).
[1] tolerance
[2] Tregs
[3] CD38
[4] liver transplantation
[5] ROS
[6] cell survival
[7] GGT1
[8] GSH
