Selective BCL-2 inhibition to promote hematopoietic mixed chimerism and renal allograft tolerance in low-dose cyclophosphamide-based conditioning regimen without myelosuppression
Toshihide Tomosugi1, Ahmad Karadagi1, Ryo Otsuka1, Katsuhiro Tomofuji1, Ivy A Rosales1, Hiroshi Sogawa1, A Benedict Cosimi1, Tatsuo Kawai1.
1Center for Transplantation Sciences, Massachusetts General Hospital, Boston, MA, United States
Purpose: We recently demonstrated the efficacy of a selective Bcl-2 inhibitor (Bcl-2i, venetoclax) in promoting mixed chimerism induction, thereby significantly reducing the dose of total body irradiation (TBI) to induce renal allograft tolerance in nonhuman primates (NHPs). This venetoclax’s effect was achieved by the selective deletion of hematopoietic stem cells within bone marrow niches, while preserving mature myeloid cells thereby avoiding leukopenia. In the current study, we evaluated venetoclax in our cyclophosphamide (CP)-based conditioning regimen.
Method: Sixteen cynomolgus monkeys underwent combined kidney and bone marrow transplantation (CKBMT) with a non-myeloablative conditioning regimen, which included CP, local thymic irradiation (7 Gy), anti-thymocyte globulin, anti-CD154 mAb, and a 28-day course of cyclosporine. Following this, no immunosuppression was administered. The total dose of CP varied among groups: 120 mg/kg in two divided doses in Group A, 150 mg/kg in Group B, and 200 mg/kg in Group C. In Groups D, peri-transplant Bcl-2i (daily venetoclax 10 mg/kg Day -4 to Day +6) was added, and the dose of CP was reduced to 50-60 mg/kg. (Table 1).
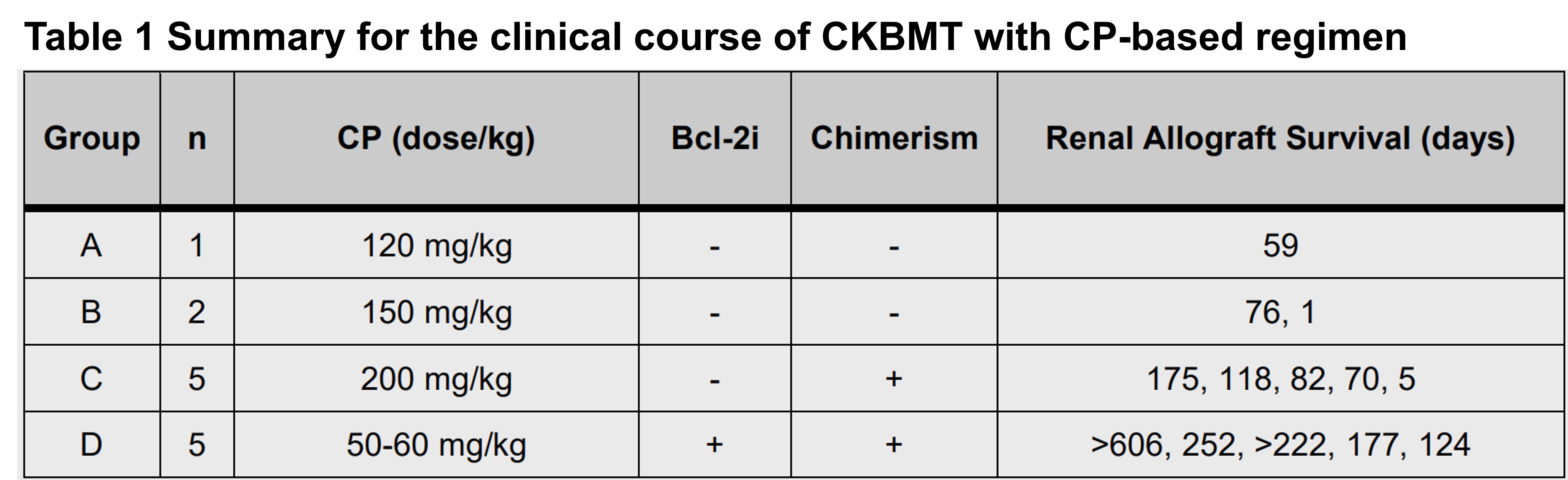
Results: Without Bcl-2i, a dose of 200 mg/kg of CP was required to induce chimerism. However, this CP dose was associated with toxicity and severe leukopenia, which resulted in poor graft survival. By adding Bcl-2i (Group D), multilineage mixed chimerism was successfully induced even with significantly reduced doses (50-60 mg/kg) of CP (Fig. 1a, 1b) with minimal leukopenia (Fig. 1c). Significantly improved immunosuppression-free renal allograft survival was achieved in Group D (p<0.006) (Fig. 1d).
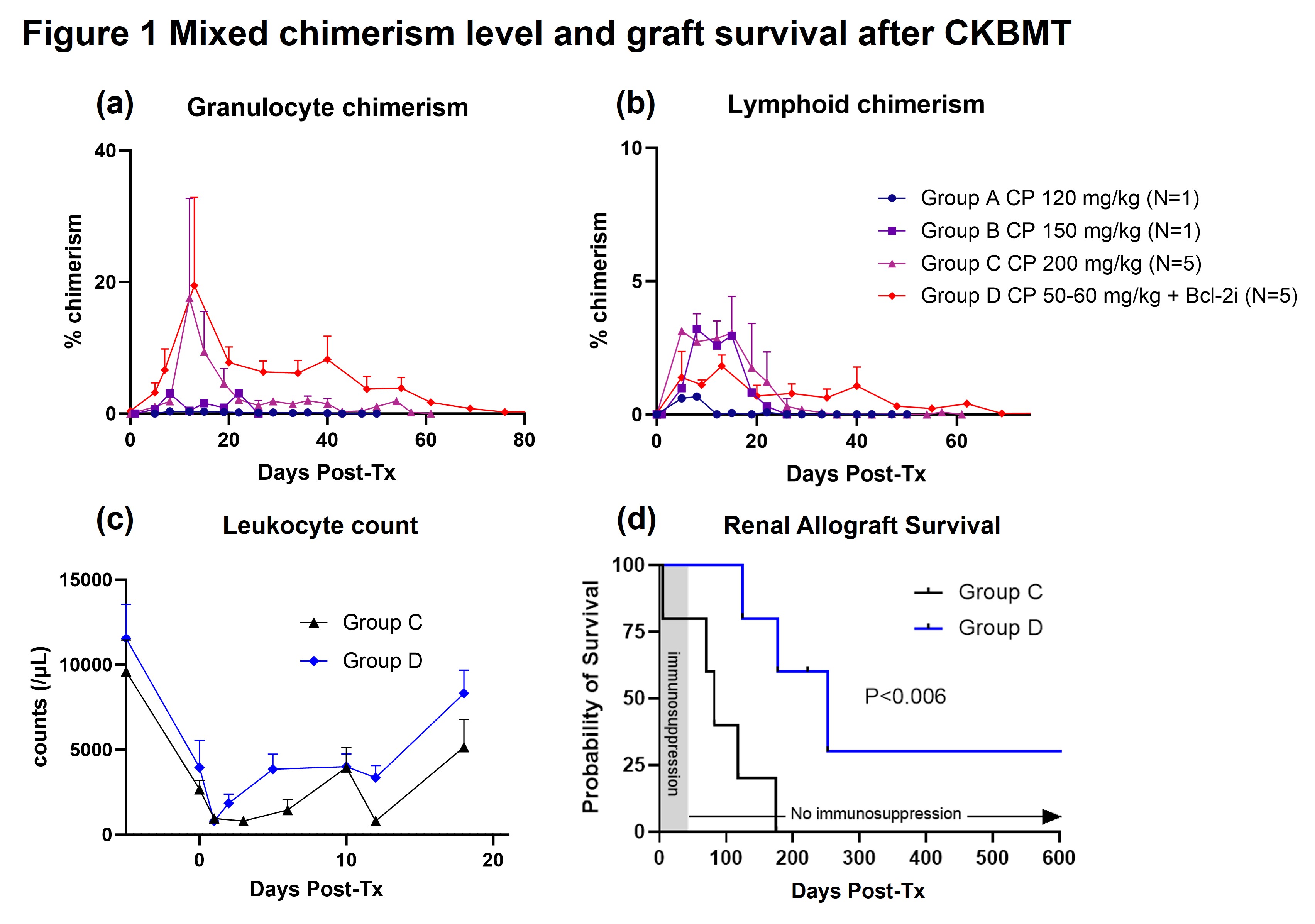
Conclusion: Bcl-2i promoted hematopoietic chimerism in a CP-based conditioning regimen without severe myelosuppression. The optimal minimum dose of CP to induce sufficient levels of chimerism to achieve allograft tolerance remains to be determined.
[1] Mixed chimerism
[2] Tolerance
[3] Cyclophosphamide
[4] BCL-2 inhibitor
