Simon N Chu, United States has been granted the TTS Scientific Congress Award
Aire-expressing cells are novel mediators of allograft tolerance in a murine heterotopic heart transplant model
Simon Chu1,2, Jiaxi Wang2, Eva Gillis-Buck1,2, Longhui Qiu3, Charles Rickert1,2, James M Gardner1,2.
1Department of Surgery, University of California, San Francisco, San Francisco, CA, United States; 2Diabetes Center, University of California, San Francisco, San Francisco, CA, United States; 3Microsurgery Core, University of California, San Francisco, San Francisco, CA, United States
Introduction: The AutoImmune REgulator (Aire) gene is pivotal in the establishment and maintenance of immune tolerance and homeostasis. Its functional significance has been established in preventing autoimmunity through upregulation of tissue-specific antigen expression in medullary thymic epithelial cells (mTECs). Recently, extrathymic Aire-expressing cells (eTACs), found in secondary and tertiary lymphoid organs, were shown to be critical for the maintenance of maternal-fetal immune tolerance. Here, we investigated the role of Aire-expressing cells in mediating transplantation tolerance utilizing a MHC class II-mismatched vascularized cardiac transplant model of chronic rejection.
Methods: We previously developed an Aire-regulated transgenic mouse line (AireDTR mice) that drives the expression of the diphtheria toxin receptor (DTR) to allow for controlled ablation of Aire-expressing cells in vivo through diphtheria toxin administration. Bm12 donor hearts (B6(C)-H2-Ab1bm12/KhEgJ) were transplanted into recipient AireDTR or wildtype mice, followed by diphtheria toxin treatment. Transplant survival was assessed by transabdominal palpation of the allograft, and cessation of heartbeat was confirmed by echocardiography. At the time of rejection, the allograft and native heart were harvested for histology and the spleen and draining lymph nodes were processed for flow cytometry.
Results: A total of 6 AireDTR and 9 wild-type mice were transplanted and followed for 90 days. Accelerated rejection was observed in AireDTR mice compared to wild-type (Log-rank test p<0.05, Figure 1B). On the day of harvest, rejected allografts appeared grossly edematous and were friable (Figure 1C). On H&E staining, rejected allografts exhibited diffuse cell infiltrate and hemorrhage, indicative of cell-mediated rejection (Figure 1D). Flow cytometry showed an increase in activated CD69+ CD4+ T cells in the spleen and draining lymph nodes of rejected grafts.
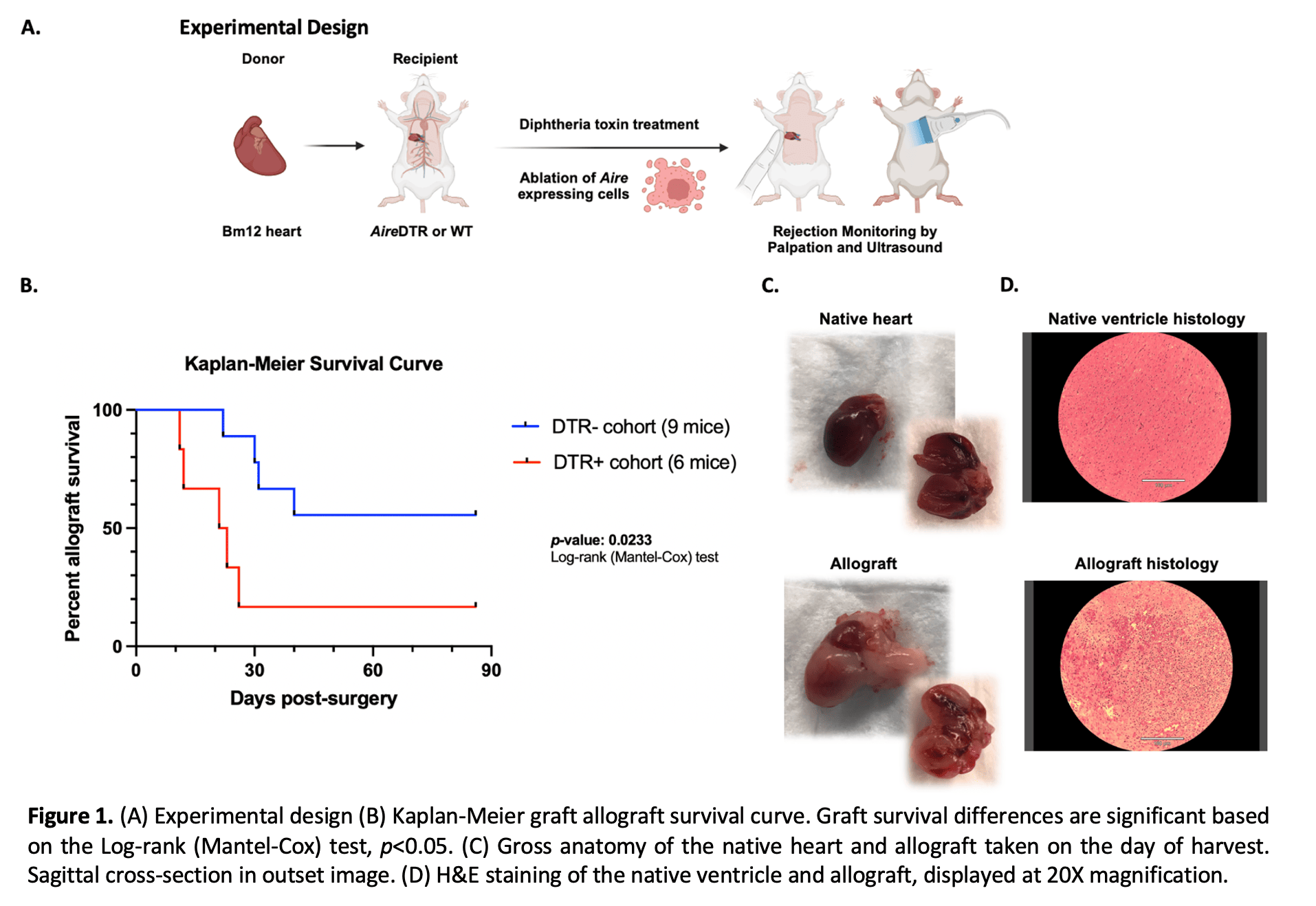
Conclusion: We demonstrate that ablation of Aire-expressing cells leads to accelerated rejection in a mouse heterotopic heart transplant model. These data support a previously undescribed role for Aire-expressing cells in maintaining immune tolerance and preventing allograft rejection. Further studies are needed to determine the immune mechanisms, particularly the relative contributions of thymic mTECs and eTACs underlying this rejection phenotype.
